
The Project Gutenberg EBook of Texas Rocks and Minerals, by Roselle M. Girard
This eBook is for the use of anyone anywhere in the United States and most
other parts of the world at no cost and with almost no restrictions
whatsoever. You may copy it, give it away or re-use it under the terms of
the Project Gutenberg License included with this eBook or online at
www.gutenberg.org. If you are not located in the United States, you'll have
to check the laws of the country where you are located before using this ebook.
Title: Texas Rocks and Minerals
An Amateur's Guide
Author: Roselle M. Girard
Illustrator: Bill M. Harris
Release Date: August 18, 2016 [EBook #52839]
Language: English
Character set encoding: UTF-8
*** START OF THIS PROJECT GUTENBERG EBOOK TEXAS ROCKS AND MINERALS ***
Produced by Stephen Hutcheson, Dave Morgan and the Online
Distributed Proofreading Team at http://www.pgdp.net

BUREAU OF ECONOMIC GEOLOGY
The University of Texas
Austin, Texas
Peter T. Flawn, Director
Guidebook 6
By
ROSELLE M. GIRARD
Sketches by Bill M. Harris

February 1964
Second Printing, April 1972
Third Printing, April 1976
Fourth Printing, May 1979
This booklet has been designed to serve as a brief, simple guide that will be of help to school children, amateur collectors, and others who are just beginning to develop an interest in the rocks and minerals of Texas. It is a companion volume to Texas Fossils by William H. Matthews III published as Guidebook No. 2 by the Bureau of Economic Geology.
Numerous present and former staff members of The University of Texas contributed time and talents to the preparation of this book, and their help is gratefully acknowledged: Peter T. Flawn, Director of the Bureau of Economic Geology, Thomas E. Brown, John W. Dietrich, Alan Humphreys, Elbert A. King, Jr., Peter U. Rodda, and others, including the late John T. Lonsdale, made many helpful suggestions; John S. Harris and Miss Josephine Casey edited the manuscript; Cader A. Shelby prepared a number of the photographs; Bill M. Harris made the illustrative sketches under the direction of James W. Macon; and Cyril Satorsky designed the cover.
Roselle M. Girard
Texas has a great variety of rocks and minerals—some are common and others are not. This book is designed to acquaint you with some of them and to tell you in a nontechnical way what they are like, some of the places where they are found, and how they are used. Although we do not know exactly how all of the rocks and minerals formed, some of the ideas about their origin are mentioned.
If you would like to learn more about rocks and minerals in general, the names of several reference books are listed on page 100. In addition, scientific reports that describe in detail many of the rocks and minerals of Texas have been published by the Bureau of Economic Geology of The University of Texas, the United States Geological Survey, and other organizations. A selected list of these reports is given on pages 100-101.
Rocks and minerals are familiar objects to all of us. We pick up attractive or unusual pebbles for our collections, we admire rocky mountain peaks, we speak of the mineral resources of our State and Nation. Rocks and minerals enter, either directly or indirectly, into our daily living. From them come the soils in which grow the grains, the fruits, and the vegetables for our food, the trees for our lumber, and the flowers for our pleasure. The iron, copper, lead, gold, silver, and manganese, the sulfur and salt, the clays and building stones, and the other metals and nonmetals that we require for our way of living were once a part of the earth’s crust.
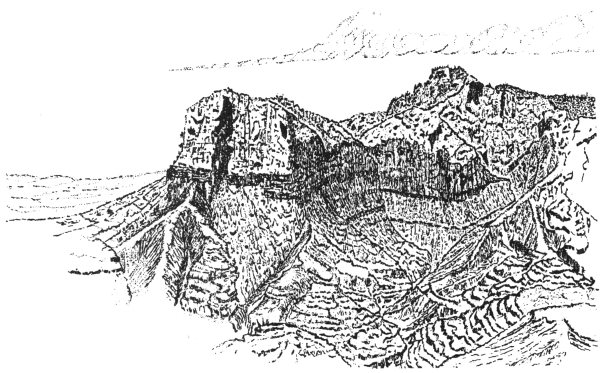
Texas’ highest mountain is Guadalupe Peak, right, with an elevation of 8,751 feet. El Capitan, left, has an elevation of 8,078 feet. These peaks in the Guadalupe Mountains in Culberson County consist largely of Capitan reef limestone, which formed during the Permian Period.
Rocks and minerals make up most of the outer layer or crust of our earth—the actual ground beneath our feet. The crust is approximately 18 to 30 miles thick beneath the continents. In general, the outermost part consists of many layers of stratified rocks, one above another. The older rocks normally make up the bottom or the deeper layers, and the younger rocks form the upper layers. Not all the layers are perfectly flat and parallel—some are lenticular (lens-shaped), some are tilted, some are partly eroded away, and some are present in one place and absent in another. Beneath the continents, the layers of rock rest on ancient metamorphic rocks and on great masses of igneous rock such as granite. These lower rocks are known as the basement.
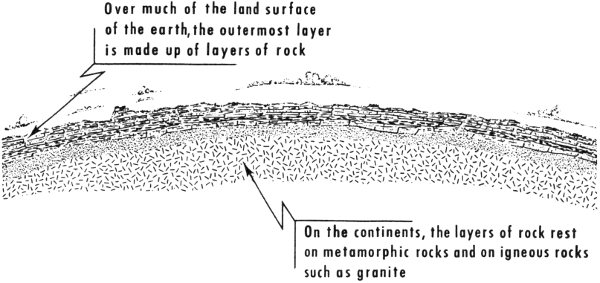
Earth’s outer crust (thickness not drawn to scale).
Over much of the land surface of the earth, the outermost layer is made up of layers of rock
On the continents, the layers of rock rest on metamorphic rocks and on igneous rocks such as granite
Those who study the earth’s crust—its origin, history, rocks, minerals, fossils, and structure—are known as geologists. The geologists who are especially interested in a particular phase of geology, as this science is called, are given special names: those who study fossils are called paleontologists; those who study minerals are called mineralogists; those who study rocks are called petrologists.
The earth’s crust is believed to be at least 3¼ billion years old. In order to deal with this vast stretch of time, geologists have divided the billions of years into various time units and have given each unit a name. The great divisions of geologic time, called eras, are Early Precambrian, Late Precambrian, Paleozoic, Mesozoic, and Cenozoic. These eras are divided into smaller units of time called periods, and the periods are divided into epochs. The [xx time scale] shows the geologic time divisions. Earliest geologic time is shown at the bottom of the scale; most recent is shown at the top.
By examining and studying the different rocks and rock layers, geologists try to discover in which unit of geologic time these rocks formed. Those rocks that formed during a period of geologic time are called a system of rocks; those that formed during an epoch are called a series. For example, the Cambrian System of rocks formed during the Cambrian Period; the Cretaceous System of rocks formed during the Cretaceous Period; the Tertiary System of rocks formed during the Tertiary Period. We are now in the younger epoch (called Recent) of the Quaternary Period of the Cenozoic Era. The rocks that are forming now are the Recent Series of rocks.
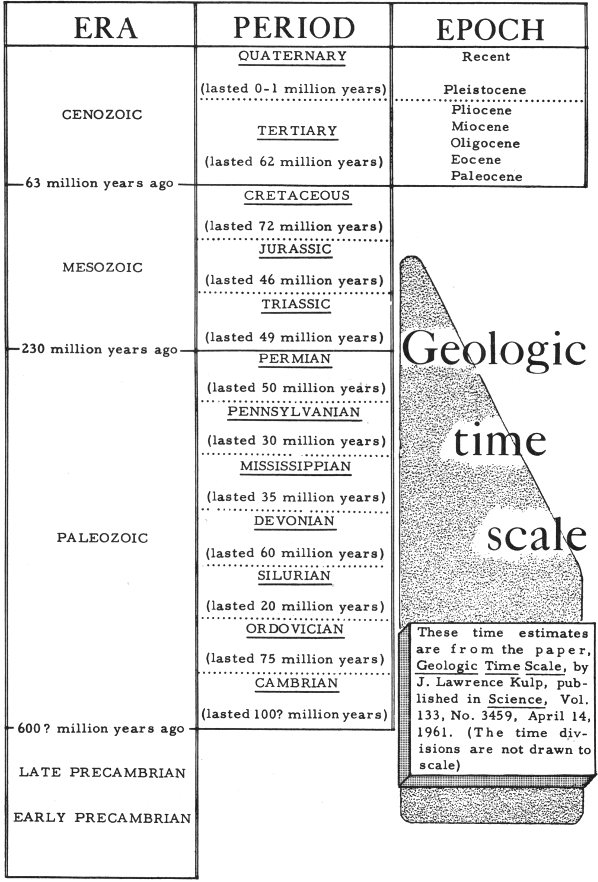
Geologic time scale
These time estimates are from the paper, Geologic Time Scale, by J. Lawrence Kulp, published in Science, Vol. 133, No. 3459, April 14, 1961. (The time divisions are not drawn to scale)
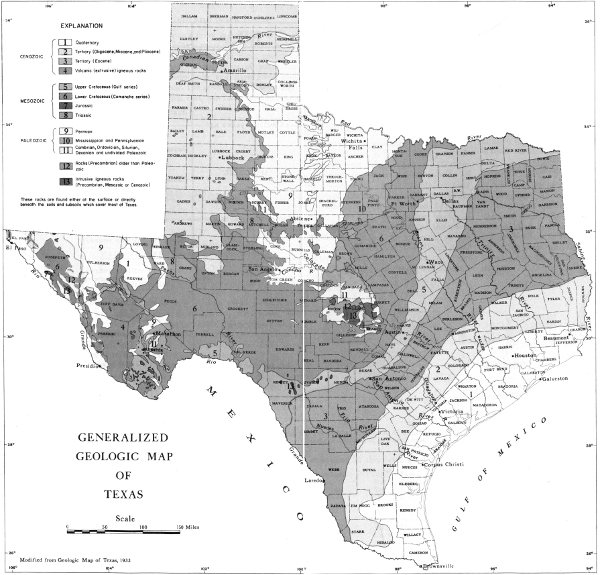
Plate 10. GENERALIZED GEOLOGIC MAP OF TEXAS
Modified from Geologic Map of Texas, 1933
These rocks are found either at the surface or directly beneath the soils and subsoils which cover most of Texas.
Geologists also subdivide rocks into lesser units. One of these, called a group, is made up of two or more formations. A formation comprises rocks or strata (layers of rock) that are recognized and mapped as a unit. Some formations consist of layers of one particular type of rock, such as limestone or shale. Formations are named after a nearby geographic locality, and in some formation names, the type of rock is included. For example, three of the Texas geologic formations are called Buda Limestone, Del Rio Clay, and Eagle Ford Shale.
The geologic map (pp. 4-5) shows the rocks that are found at the surface in Texas. Some of these are extremely old. Some, geologically speaking, are very young.
Although rocks and minerals are often mentioned together, and to some people they have similar meanings, geologists make a distinction between the two words. In general, rocks are made up of minerals, and minerals are made up of chemical elements.
The chemical elements include oxygen, silicon, calcium, sulfur, carbon, gold, silver, and many others. There are 90 naturally occurring elements. Each is made up of molecules that consist of only one kind of atom. Chemical elements may either be combined with each other or occur alone. They are the building blocks of our world for they make up all the gases, all the liquids, all the minerals, all the plant and animal life, and all the other physical matter. Some of the chemical elements that occur in the rocks and minerals mentioned in this book are listed below.
| Aluminum | Al |
| Barium | Ba |
| Beryllium | Be |
| Boron | B |
| Calcium | Ca |
| Carbon | C |
| Cerium | Ce |
| Chlorine | Cl |
| Copper | Cu |
| Fluorine | F |
| Gold | Au |
| Hydrogen | H |
| Iron | Fe |
| Lead | Pb |
| Magnesium | Mg |
| Manganese | Mn |
| Mercury | Hg |
| Molybdenum | Mo |
| Oxygen | O |
| Potassium | K |
| Silicon | Si |
| Silver | Ag |
| Sodium | Na |
| Strontium | Sr |
| Sulfur | S |
| Thorium | Th |
| Tin | Sn |
| Uranium | U |
| Vanadium | V |
| Yttrium | Y |
| Zinc | Zn |
| Zirconium | Zr |
We can compare the chemical elements to the letters of our alphabet. The letters, like the chemical elements, are fundamental building blocks, and they can be brought together in various combinations to form words.
A mineral can be compared to a word of our language. We combine letters to form a word, and nature combines certain chemical elements to form each particular mineral. For example, calcite, a mineral that is abundant in Texas, is always made up of the same proportions of the same three elements: calcium, carbon, and oxygen.
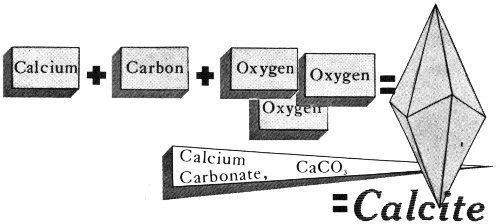
A mineral is made up of chemical elements. The mineral calcite, for example, always consists of the same proportions of calcium, carbon, and oxygen.
Each mineral has its own characteristic internal structure and other properties. At ordinary temperatures, nearly all the minerals are solids rather than gases or liquids. (Water and mercury are the principal exceptions.) In addition, minerals are inorganic rather than being composed of plant or animal matter.
When a single chemical element is found alone in nature as a solid, it is considered to be a mineral, too. Gold, silver, copper, lead, and sulfur are some of the chemical elements that can occur alone as solid minerals. When they occur this way, we refer to them as native silver, native copper, or native sulfur. Although the element mercury is a liquid rather than a solid at ordinary temperatures, it too is a mineral when it occurs alone in nature. It is then called native mercury.
We have already compared the chemical elements to the alphabet and the minerals to words. We can now go a step further and compare rocks to sentences. We put words together to make sentences; nature puts minerals together to make rocks. A sentence does not have to be made up of a definite number of words, nor does a rock have to be made up of a definite number of minerals. Some rocks, such as granite, may be composed of several minerals. Others, such as dolomite and rock gypsum, consist of only one mineral.
Minerals do not lose their identities when they make up a rock. Instead, they are merely associated together in varying proportions. Some rocks, as we will find later, instead of being composed of the minerals themselves, are made up of fragments of earlier-formed rocks.
Ordinarily, we think of rocks as hard and solid substances, such as limestone and granite, but some geologists consider loose and uncemented materials, such as sand, gravel, or volcanic ash, to be rocks also. The words sediments or deposits are often used to describe this uncemented or loose material.
Rocks are commonly grouped, according to how they formed, into three great classes known as igneous, metamorphic, and sedimentary.
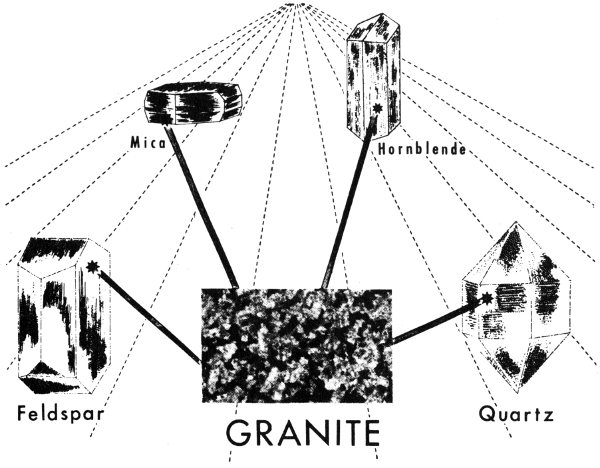
A rock is made up of minerals. The igneous rock granite, for example, consists chiefly of quartz and feldspar; other minerals such as mica and hornblende are commonly present.
Igneous rocks result from the cooling of hot, molten rock material or magma. Magma that reaches the surface through volcanoes is called lava. Magma comes from deep within the earth and is made up of a mixture of molten mineral materials. Igneous rocks have been forming throughout the geologic past and are still forming today. We can understand how they form when we look at pictures of hot, molten lava flowing from volcanoes, such as Mauna Loa in Hawaii. As lava cools, it hardens into rock.
The igneous rocks that form on the earth’s surface are called extrusive or volcanic igneous rocks. When magma flows to the surface, it cools and hardens quickly. The mineral grains that form during this fast cooling may be too small to be distinguished from each other. Some lava cools too quickly for minerals to crystallize—then the rock is volcanic glass.
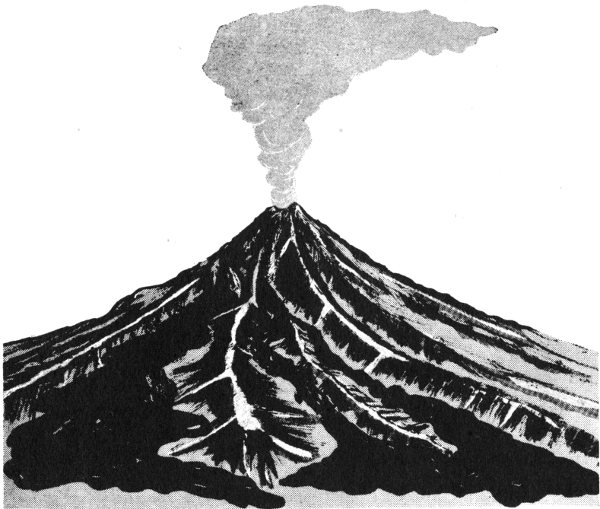
Extrusive igneous rocks form at the earth’s surface from lava that cools and hardens relatively quickly.
No volcanic igneous rocks are forming in Texas now. However, during Tertiary time, in the Big Bend area and in other parts of the Trans-Pecos country of west Texas, lava came to the surface and hardened. (The physiographic outline map, p. 42, shows where these areas are located.)
The cooling and hardening of hot, molten magma also takes place below the earth’s surface. Here, the magma cools slowly to form rocks made up of mineral grains that are large enough to be readily visible. These rocks are known as intrusive igneous rocks. We know that they are present below the surface in Texas because of wells drilled in many areas of the State. In Pecos County, a well reached granite, an intrusive igneous rock, at a depth of 16,510 feet. Other wells in Texas have reached the granite basement rocks at much shallower depths. But not all intrusive igneous rocks in Texas are found 10 underground. In the Trans-Pecos country of west Texas, in the Balcones fault zone, and in the Llano uplift of central Texas, some are now seen at the surface. They, like all intrusive rocks, were formed below the ground, but earth’s processes of uplift and erosion have gradually uncovered them.
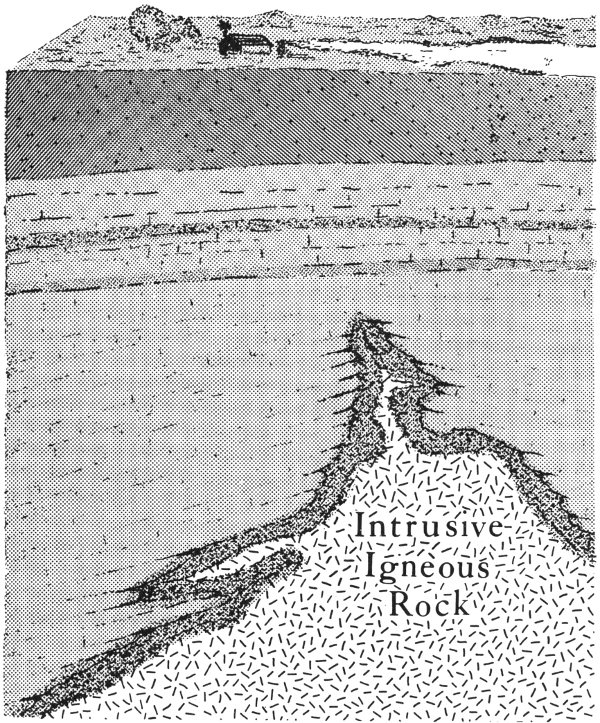
Intrusive igneous rocks form from molten rock material (magma) that cools and hardens beneath the earth’s surface.
Sedimentary rocks are made up of sediments, which are rock and mineral grains that have come from weathered rocks of all kinds. Rocks are weathered when water, ice, snow, wind, and other agents cause them either to dissolve, as table salt does when put in water, or to break apart, as old pavement commonly does.
Some of the broken-down rocks, along with associated plant and animal matter, develop into soils. When you examine soil with a magnifying glass, you may be able to see some of the small rock and mineral grains that still remain in it. 11 Some soils have formed on top of the rocks from which they came, and some have been moved in from another place.
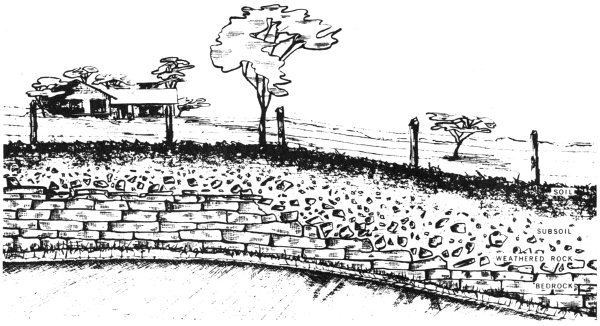
Soils develop from weathered rock and associated organic material.
Water and wind not only weather the rocks and soils but also move the weathered materials (the sediments) and deposit them in other places. Whenever you see a dust or sand storm, or a muddy creek or river, you are observing the movement of sediments by wind and water to other land areas or to the sea. The combination of weathering and movement is called erosion.
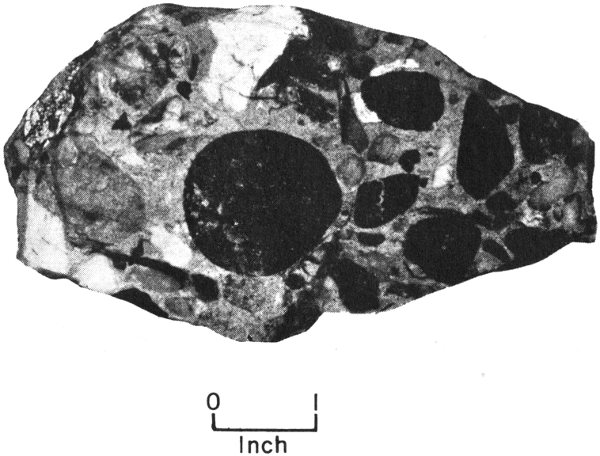
Conglomerate from Webb County, Texas, is composed of rounded gravel that has been cemented together.
Some of the rock fragments carried by water are still fairly large when they reach their destinations. On the basis of size, they are called boulders, cobbles, pebbles, and granules. Loose deposits of these larger-size sediments make up what is 12 known as gravel. Nature cements gravels together to form rocks such as conglomerates (made up of rounded gravel) and breccias (made up of sharp-cornered gravel).
The finer sediments are called sand, silt, mud, and clay. When cemented, the sand grains become sandstones, the silt particles become siltstones, and the mud and clay particles become shale. The sedimentary rocks that are made up of these rock fragments are called clastic or fragmental rocks.
As they are weathered, some rocks dissolve and go into solution. For example, a number of the Texas creeks and rivers carry calcium carbonate in solution because they flow through areas where limestone rocks, which consist mostly of calcium carbonate, are being weathered. (Water that contains a large amount of dissolved rock material is called hard water.)
Some of the waters containing dissolved rock material seep through loose sediments where the dissolved material may come out of solution and form a cement, which binds the sediments together. For example, when loose sand sediments are cemented, they form sandstone. Three of the most common cements are iron oxide, calcium carbonate, and silicon dioxide, although a number of other materials also serve as cements.
Dissolved rock materials come out of solution not only to serve as cementing agents but to form the chief mineral of some sedimentary rocks as well. Sedimentary rocks of this kind form mostly in lakes and seas into which much dissolved material is carried by rivers. When the dissolved material comes out of solution, it is said to be precipitated and the mineral sediments it forms are the chemical sediments. Some limestones originate this way. You can see examples of precipitated materials by noting the crust-like deposits that form inside some water pipes and teakettles, as dissolved material in the water comes out of solution.
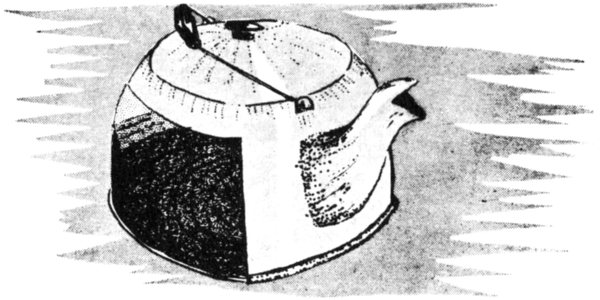
Precipitated sediments are commonly observed lining a teakettle.
The dissolved rock material can come out of solution in another way. Some plants and animals are able to take dissolved calcium carbonate out of the sea water and use it to build their shells and other structures. Some of these organisms, such as corals and algae, can grow upward from the sea floor in large groups to form reefs that later become reef limestones. Other limestones are made up of the remains of plants and animals that collect on the sea floor and become cemented together.
Metamorphic rocks come from earlier-formed rocks that have undergone a change or a metamorphosis. All igneous and sedimentary rocks, and earlier-formed metamorphic rocks too, can be changed, without being moved to some other place, 13 into new and different rocks. As they are changed, they may become harder, new minerals may form, and they may look entirely different. For example, granite, an igneous rock, can be changed into the metamorphic rock known as gneiss; limestone, a sedimentary rock, can be changed into marble; shale, a sedimentary rock, can be changed into slate. These changes occur because the earth is a big and complex chemical system. The agents that bring about these changes, which always occur below the surface of the earth, are heat, pressure, and fluids—both liquids and gases. Several different kinds of change or metamorphism can take place.
Some of the changes occur because the rocks are at great depths. As more and more younger rocks are deposited on top of them, the older rocks become deeply buried. The great thicknesses of younger rocks are heavy, and they squeeze and press down on the rocks beneath them. The deeply buried rocks are also hotter than surface rocks. In general, the temperature increases about 1° Fahrenheit for each 50 feet of depth below the surface. The change of deeply buried rocks into new rocks by pressure and heat is known as static metamorphism.
Another method of change or metamorphism involves molten igneous rock material. When hot magma moves up through rocks, it not only heats and pushes them, but it also may soak them with liquids and gases, causing the nearby rocks to change into new rocks, by a process called contact metamorphism.
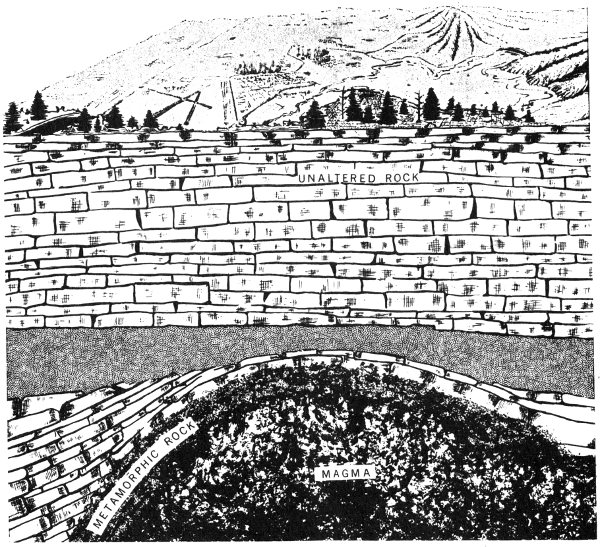
Some rocks are altered by heat and fluids when they are invaded by hot magma in a process called contact metamorphism.
Still another rock-changing process is one that is associated with mountain building. When mountains are formed, heat and great pressures develop deep within the earth’s crust. The flat layers of rock are then slowly pushed and squeezed so that they bend up into arches, fracture, or slide over each other. These forces cause great changes in the rocks in widespread areas. This process of change is known as dynamic metamorphism.
Rocks are made up of minerals. In addition, minerals are associated with rocks in other ways. For example, minerals fill or coat cracks and cavities that have developed in some of the rocks. Minerals are either crystalline or amorphous.
Most minerals are crystalline. In crystalline minerals, combinations of atoms are arranged in ordered patterns, which are repeated over and over. This orderly internal structure of atoms is a characteristic of each crystalline mineral, as mineralogists are able to determine by using X-rays and special microscopes.
When a mineral occurs as a well-formed individual crystal, it has a definite, precise shape. The kind of crystal shape it has depends on its own type of crystalline internal structure. A well-formed crystal has smooth, flat, outer surfaces called crystal faces, which are arranged together to form prisms, cubes, pyramids, and many other geometric shapes. For example, quartz, a common Texas mineral, is commonly found as a six-sided, prism-shaped crystal that is topped by pyramid-like forms. Pyrite, another common mineral, occurs as cube-shaped crystals. We can identify some minerals more readily by learning to recognize their crystal shapes.
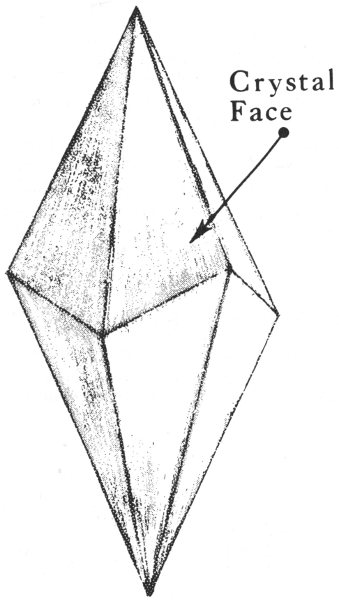
A scalenohedron, one of the many crystal forms of calcite.
A crystalline mineral commonly forms under conditions that do not permit it to become a well-shaped crystal. Although the mineral may show a few crystal faces, it does not have a complete crystal shape and so is described as massive, or is said to occur in masses. Some of the minerals that make up rocks occur as crystalline masses. For example, calcite is a crystalline mineral that occurs in the metamorphic rock marble without its normal crystal shape.
Many crystalline minerals occur as incomplete and imperfect crystals that are grouped together in various arrangements. If these incomplete crystals are arranged around a common center like the spokes 15 of a wheel, they are said to be radial or radiated. If the groups of incomplete crystals look like bundles of strings or fibers, they are described as fibrous. If they are in rounded masses that resemble bunches of grapes, they are called botryoidal. If they look like fish scales, they are described as scaly. Some crystalline minerals are made up of tiny grains that are grouped together like the grains in a lump of sugar. A mineral occurring in this way is described as granular. More descriptions of crystalline minerals are found in the section on Texas rocks and minerals (pp. 43-98).
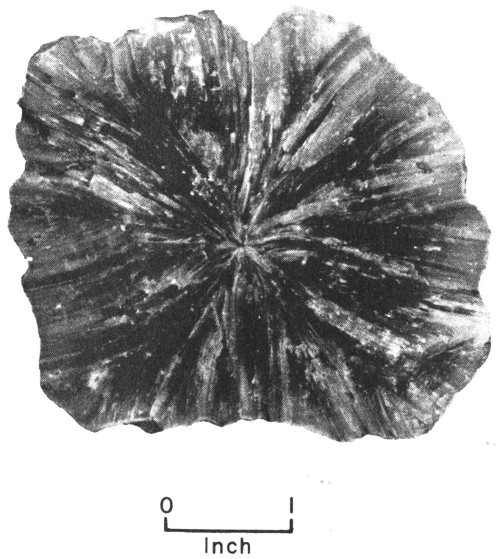
Barite specimen showing radial form.
An amorphous mineral, unlike a crystalline mineral, does not have a definite, orderly arrangement of its atoms. Because of this lack of internal structure, the mineral occurs in masses that have no regular geometric shapes, and it has no crystal form of its own. Only a few minerals are amorphous.
We use our senses of sight, hearing, smell, touch, and taste to become aware of the world around us. For example, we recognize a flower by noting its color, its fragrance, and the texture, shape, and arrangement of its petals. These are some of its characteristic properties. A mineral also has distinguishing properties, among them color, luster, and hardness, which help us identify it. Some minerals have a single outstanding property, such as the magnetism of magnetite, that makes them easier to recognize. But to identify most minerals, we need to determine not just one, but several properties.
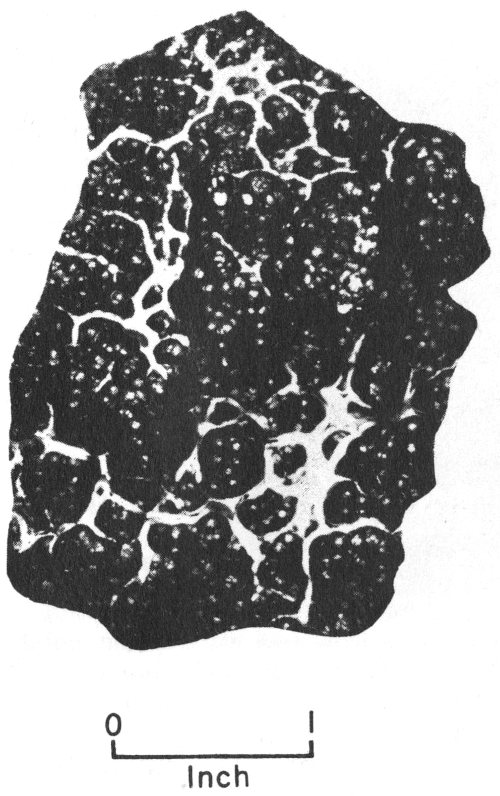
Chalcedony showing botryoidal form.
Color is one of the properties we notice first. The color of some minerals is always the same, and it helps us to identify them. But it is not a dependable property to use in identifying all minerals, because some contain impurities that change or hide the real color.
The luster is the way the surface of a mineral reflects light. The luster of a mineral may be nonmetallic, submetallic, or metallic. Mineral metals such as gold, silver, galena, and pyrite have a metallic luster. A few minerals have a luster that is almost, but not quite metallic—their luster is submetallic. A mineral with a nonmetallic luster may look vitreous (glassy), silky, resinous (like resin), greasy, earthy (dull), pearly, or adamantine (brilliant).
Some minerals allow light to pass through them; others do not. A mineral is transparent if you can see both light and objects through it, as through clear glass. If you can see only light, but no objects, as through frosted glass, the mineral is translucent. When you hold an opaque mineral up to the light, it looks dark. No light at all comes through it, even through the thin edges.

Transparent mineral.
Some minerals are soft and can be scratched easily. Others, which are harder, are resistant to scratching. To measure a mineral’s hardness, we try to find out which substances will scratch it and which substances will not scratch it. To do this in a general way, several ordinary objects—such as a fingernail, a copper penny, a pocket knife, a piece of window glass, and a steel file—can be used. For a more exact way of testing hardness, we can use ten minerals that make up what is known as Mohs scale. Each mineral in this scale has a different hardness, and each one has been given a number that represents its hardness. For example, talc, the softest mineral in this scale, is given a hardness of 1. Gypsum, the next softest mineral in the scale, has a hardness of 2. Diamond, the hardest mineral known, is given the top hardness of 10 in this scale. These ten minerals are listed below. Alongside 17 them are five common objects with their hardnesses.
| 1—Talc | |
| 2—Gypsum | Fingernail—slightly over 2 |
| 3—Calcite | Copper penny—about 3 |
| 4—Fluorite | |
| 5—Apatite | Pocket knife—slightly over 5 |
| 6—Orthoclase | Window glass—5½ |
| 7—Quartz | Steel file—about 6½ |
| 8—Topaz | |
| 9—Corundum | |
| 10—Diamond |
Suppose, for example, that a mineral can be scratched by fluorite, which has a hardness of 4 on Mohs scale, but cannot be scratched by calcite, which has a hardness of 3. We then know that this mineral is softer than fluorite, but harder than calcite; therefore, it has a hardness of about 3½. In the same way, if a mineral can be scratched by a pocket knife, which is slightly more than 5 in hardness, but not by a copper penny, which has a hardness of about 3, we know then that its hardness is between 3 and 5.
The streak is the mark, made of fine powder, that a mineral leaves as you rub it across a streak plate. A streak plate is a flat piece of white tile or porcelain that has a dull, unglazed surface. The streak plate is about as hard as quartz, which is 7 on Mohs scale, and you will not be able to use it for minerals that have a greater hardness. For these, you can obtain the powder by scratching the mineral or by crushing a small piece of it.
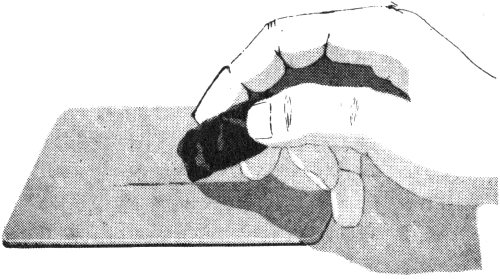
A streak plate is used to determine the color of the streak or powder of a mineral.
The color of the streak or powder is extremely helpful in identifying some minerals. For example, hematite is a mineral that may be any one of several different colors, but its streak or powder is always reddish brown.
As they break, some crystalline minerals always split along a smooth, flat surface. This property is known as cleavage. Some cleavages are smooth and perfect; others are not so perfect. The cleavage surfaces, because of the mineral’s crystalline internal structure, are parallel to possible crystal faces, even though the mineral itself may occur as a crystalline mass without a perfect crystal shape.
Some minerals will cleave in only one direction; some, in several directions. For example, galena, a mineral found in Texas, has perfect cubic cleavage. It cleaves in three directions that are at right angles to each other. These cleavage directions are parallel to possible cubic crystal faces, and some of the cleavage fragments are cubes.
A few minerals sometimes show a kind of false cleavage known as parting. Parting, unlike cleavage, is not constant and does not occur in every specimen of a particular mineral. For this reason, it is not a very dependable means of identification.
Minerals also break in another way. When the break is in a different direction from that of the cleavage or parting, it is known as the fracture. A fracture is called conchoidal if the mineral’s broken surface is curved like the inside of a spoon or shell. Thick pieces of glass break with this conchoidal fracture. A fracture is described as hackly if the broken surface has sharp, jagged edges; as even, if the surface is generally flat; and as uneven, 18 if it is rough and not flat. If the mineral breaks into splinters, its fracture is called splintery.
The specific gravity is a measure of whether a mineral is heavy or light. It is a comparison of the weight of a piece of the mineral with the weight of an equal volume of water. The mineral quartz, for example, has a specific gravity of 2.65. This means that a piece of quartz is a little more than 2½ times as heavy as an equal volume of water. Accurate measurements of specific gravity can be made in a laboratory. You can, however, learn to estimate specific gravities just by lifting various minerals and judging whether they are heavy or light.
This is a property that depends on the chemical composition of the mineral. Carbonate minerals, which contain (in addition to at least one other element) three parts of oxygen and one part of carbon, can be tested with dilute hydrochloric acid. When a drop or two of this acid is put on a carbonate mineral such as calcite (calcium carbonate, CaCO₃), the acid begins to bubble and fizz. The fizzing or effervescence is caused by the carbon dioxide gas that is formed when the acid and mineral come in contact with each other. This test is also helpful in identifying rocks, such as limestone and marble, that contain carbonate minerals.
Beautiful mineral deposits occur in some natural caves. Deposits that look like icicles, called stalactites, are found hanging from the ceiling of a cave. Other deposits, stalagmites, are like the stalactites except that they jut upward from the floor. Columns are formed from stalactites and stalagmites that have joined together. In addition, some caves contain sheet-like deposits that are spread along the ceiling, floor, and walls. These deposits are called flowstone. Calcite is one of the minerals that commonly form cave deposits.
Just a few of the caves in Texas contain these deposits. They occur mostly in the limestone rocks that are south and southwest of the Llano uplift area of central Texas. Some of the commercial caves that contain good examples of calcite deposits are located near Boerne in Kendall County and near Sonora in Sutton County. Calcite deposits also occur in Longhorn Cavern, a large cave located in the Longhorn Cavern State Park of Burnet County. These caves were formed by underground waters that moved through cracks and pores in the limestone rocks and dissolved passageways in them. After the cave passages were made, water containing dissolved calcium carbonate dripped into the cave. As it evaporated, this water left behind a deposit of calcium carbonate—the mineral calcite.
You can better understand how the cave deposits are formed by watching icicles grow in wet, freezing weather. First, small hanging drops of water freeze, and a small icicle forms. Then, as more water drips 19 over it and freezes, the icicle grows longer and wider. Some of the water drips completely over the icicle and falls to the ground. There, it either freezes into a sheet of ice, or it begins to build upward to form an upside-down icicle. The water dripping down in the caves evaporates instead of freezing, and in doing so it leaves behind a deposit of calcite.
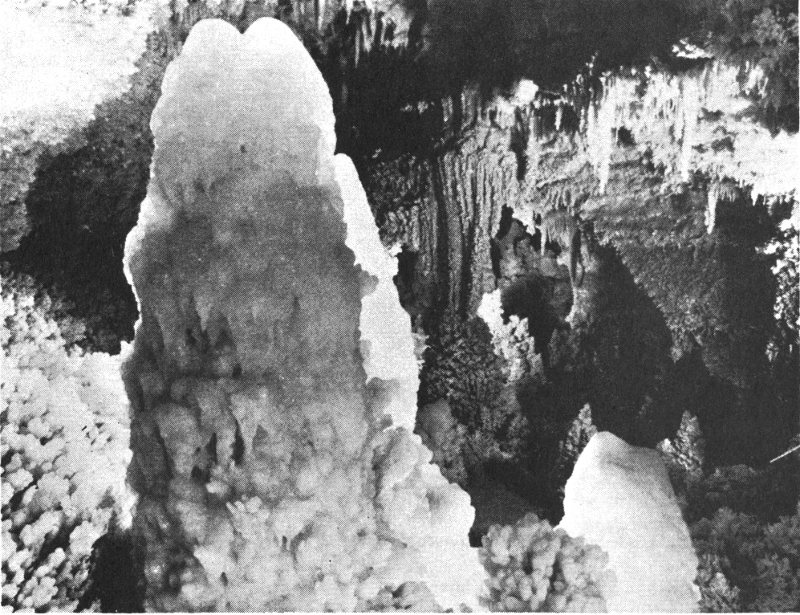
Calcite stalactites and stalagmites in the Caverns of Sonora, Sutton County, Texas. Photograph courtesy of the Travel and Information Division of the Texas Highway Department.
Limestone, shale, and other sedimentary rocks commonly have scattered throughout them masses of other rocks and minerals, such as limonite, chert, and pyrite. These masses are called concretions. Concretions may be round or oval, or they may have odd, irregular shapes. They—such as some of the limonite concretions of east Texas—even may look like gourds or sweet potatoes. Concretions generally are harder than the surrounding rocks. Some are smaller than peas, but others are several feet wide. (The word nodule is used to describe small, rounded concretions as well as other small, rounded mineral occurrences.)
It is believed that some concretions form at the same time as the rocks in which they occur. Other concretions develop after the rocks themselves have formed. These are deposited by underground water that contains dissolved mineral matter. The water seeps through the rocks and deposits mineral matter around an object in the rock, such as a fossil or a grain of sand, to form a concretion.
Geodes are rounded, generally hollow masses that occur mostly in limestones. They are scattered through the rocks and can be lifted or dug out. Some geodes are as small as walnuts, and some are as 20 large as basketballs. Most of them have a rough, dull-looking outer surface. If you break geodes open, you will find that many are lined with beautiful crystals of calcite, celestite, or quartz that point inward toward the hollow center.
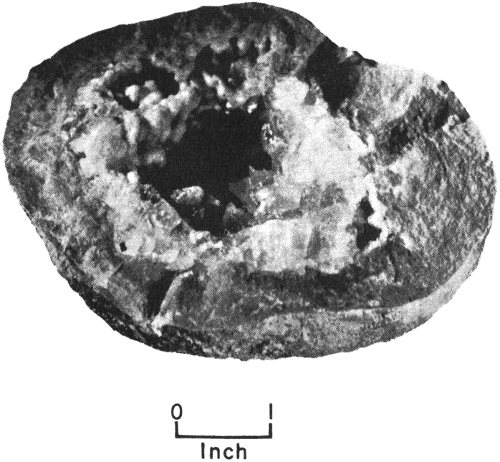
Calcite geode found in Lower Cretaceous strata of western Travis County, Texas.
It is thought that a geode forms when water, carrying dissolved mineral material, seeps into a cavity in the rock, then deposits the mineral material as a lining in the cavity. This lining becomes the outer part of the geode. Thus a geode—unlike a concretion, which grows from the center outward—forms from outside to inside.
Some of the Lower Cretaceous limestone rocks of Travis, Williamson, and Lampasas counties contain calcite and celestite geodes. Celestite geodes have also been found in Permian rocks in parts of Coke, Fisher, and Nolan counties.

Petrified wood from Texas Gulf Coastal Plain.
We often find some minerals occurring as petrified wood. (Petrified wood includes silicified wood, opalized wood, agatized wood, and carbonized wood.) Petrified wood forms when plant material, such as a tree or a bush, is replaced 21 by a mineral. It is formed by underground water carrying dissolved mineral matter. As this water seeps through sediments in which the plants are buried, it gradually deposits agate, chalcedony, calcite, opal, chalcocite, or some other mineral in the place of each fiber of the wood. By this slow change from plant to mineral matter, the original shape and structure of the wood remain unchanged.
Petrified wood is commonly found in some of the Tertiary, Permian, and Lower Cretaceous rocks of Texas. (See Opal, Quartz, Copper Minerals, pp. 78, 84, 52).
Perhaps you would like to start your own collection of rocks and minerals. For this purpose you will need a hammer (a prospector’s hammer with a pick on one end of it is a good tool), some newspapers to wrap around the specimens to keep them from breaking, and a cloth bag in which to carry the specimens.
Prospector’s hammer.
Before you start to collect, be sure to ask the owner’s permission to go on his property. If he agrees to let you come on his land, be careful about closing gates, and do not leave holes into which his livestock might step and be injured. Look out for snakes. Plenty of rattlers, copperheads, and moccasins are still left in Texas. And, incidentally, collecting is not allowed in State or National parks.
To identify the rocks and minerals that you collect, you probably will need several articles with which to make simple tests. The following can be easily obtained:
1. A pocket knife, a copper penny, a piece of window glass, a steel file, and a piece of quartz to test the hardness. If you prefer to use a group of minerals of known hardness, such as those of Mohs scale described on pages 16-17, you can either collect your own or buy a prepared set from a mineral supply house.
2. A streak plate to test the color of the mineral’s streak. Mineral streak plates can be purchased, or a piece of unglazed tile can be used.
3. A magnifying glass to examine small cleavage surfaces, crystals, and rock grains. A number of different kinds can be bought, from the simple reading glass to the precisely made hand lens. A lens with ten-power magnification is good for general use.
4. A small magnet to test whether or not a mineral is magnetic.
5. Dilute (10%) hydrochloric acid (HCl), also known as muriatic acid, to test carbonate rocks and minerals. You can buy a small bottle at a drug store. Be extremely careful in handling this acid, and keep it away from small children—it is a POISON. If you spill any on yourself, it will burn your skin and eat holes in your clothes.
Hand lens.
The rock and mineral identification charts on pages 24-41 will help you to make the simple identification tests in a methodical way.
It is a good idea to have some system of labeling your rock and mineral specimens. Some collectors carry note paper with them on field trips. Then they can write down the location and, if possible, the name of the rock or mineral. This information is either wrapped with the specimen or stuck to it with tape. One way to label large collections is to put a small spot of paint or fingernail polish on each of the rock and mineral specimens. When the paint has dried, a number can be written on it in black India ink. Then, on a file card, the name and the number of the specimen can be written, together with the place where it was found, the date of collection, and the name of the collector.
To help you identify them, various Texas rocks and minerals are listed together in the following charts according to properties that they have in common. Although useful, the identification charts may not always give you perfect results. For example, hardness, which is used as a guide, is not to be completely relied upon in the identification of rocks.
The charts on the following pages pertain only to the rocks and minerals that are described in this book. It is quite possible that you will find rocks and minerals in Texas that are not included in these charts.
If you find a rock or a mineral that you are unable to identify, you can check your local library for reference books that may aid you (several such references are noted on pages 100-101). If you need further help, possibly the science teacher at a nearby public school will be able to identify the specimen for you. Or if a college or university is located in your area (especially one that has a department of geology), you can obtain help there. In Texas, the Bureau of Economic Geology is a mineral information center. Most other states have similar geological research and public-service organizations. Other sources of information might be the gem and mineral societies that are found in a number of communities. Many of the members of these organizations are experts in the identification of rocks and minerals.
In the mineral identification charts (pp. 26-38), the minerals have been grouped, first of all, on the basis of luster: the first group includes the minerals that appear metallic and almost metallic (submetallic); the second group includes those that appear nonmetallic. Next, the minerals have been arranged within the two groups according to color.
After you have determined the luster and the color of an unknown mineral, turn to the Key to Mineral Identification Charts on page 25. It will direct you to the proper mineral chart.
Mineral Charts 1 through 5, which include the minerals of various colors with metallic and submetallic lusters, are subdivided according to the hardness of the minerals. To determine the hardness of a mineral that has one of these lusters, you can make the following tests:
1. Will the mineral readily leave a mark on paper?
2. If it will not readily leave a mark on paper, will an ordinary pocket knife scratch it?
3. Is it too hard to be scratched by an ordinary pocket knife?
Mineral Charts 6 through 15 are for the nonmetallic minerals of various colors. They, too, are subdivided according to the hardness of the minerals, as follows:
1. Can the mineral be scratched by a fingernail?
2. If it cannot be scratched by a fingernail, can it be scratched by a copper penny?
3. If it cannot be scratched by a copper penny, can it be scratched by an ordinary pocket knife?
4. If it cannot be scratched by an ordinary pocket knife, can it be scratched by a piece of quartz?
5. Is it too hard to be scratched by quartz?
When the luster, color, and hardness of a mineral have been determined, you may find that several minerals on the charts fit the description. To narrow your choice, you can then test other properties of the mineral. Notice the “remarks” column on the charts. In it, is mentioned anything that is distinctive about the mineral.
For more complete mineral identification lists and tables, you can use textbooks, such as Dana’s Manual of Mineralogy, revised by C. S. Hurlbut, Jr., or Mineralogy, by E. H. Kraus, W. F. Hunt, and L. S. Ramsdell.
If the mineral has a metallic or submetallic luster,
| and is: | Consult Mineral Chart |
| white | 1 |
| gray | 2 |
| yellow | 3 |
| brown | 4 |
| black | 5 |
If the mineral has a nonmetallic luster,
| and is: | Consult Mineral Chart |
| white | 6 |
| gray | 7 |
| yellow | 8 |
| brown | 9 |
| black | 10 |
| green | 11 |
| blue | 12 |
| red or pink | 13 |
| purple or violet | 14 |
| colorless | 15 |
| Chart No. | Mineral | Streak | Remarks | Hardness | |
| 1. | METALLIC luster, WHITE color | ||||
|---|---|---|---|---|---|
| A. | Does not readily leave mark on paper but can be scratched by ordinary pocket knife | ||||
| Native silver | Shiny silver white, unless tarnished | Silver-white color that tarnishes to gray, black, or yellowish brown; heavy; can be flattened when hit with hammer | 2½-3 | ||
| 2. | METALLIC or SUBMETALLIC luster, GRAY color | ||||
| A. | Will leave mark on paper | ||||
| Argentite | Shiny, blackish to lead gray | Lead-gray color that tarnishes to dull black; knife cuts it smoothly; heavy; may occur as masses and coatings | 2-2½ | ||
| Galena | Grayish black | Shiny lead-gray color; heavy; cube-shaped fragments and crystals | 2½ | ||
| Graphite | Black | Steel-gray color; greasy feel; very soft; splits into thin flakes | 1-2 | ||
| B. | Does not readily leave mark on paper but can be scratched by ordinary pocket knife | ||||
| Chalcocite | Grayish black | Shiny lead-gray color that tarnishes to dull black; knife cuts it smoothly; may have black sooty coating; commonly occurs as compact or granular masses | 2½-3 | ||
| Hollandite | Black | Silvery-gray color; may occur as rounded masses | 4-6 | ||
| C. | Cannot be scratched by ordinary pocket knife | ||||
| Braunite | Steel gray or black | Dark steel-gray color and submetallic luster | 6-6½ | ||
| Hematite | Dark reddish brown | Steel-gray color; commonly occurs as granular or compact masses; shiny, scaly variety is specular hematite; notice streak | 5½-6½ (may be softer) | ||
| Hollandite | Black | Silvery-gray color; may occur as rounded masses | 4-6 | ||
| 3. | METALLIC luster, YELLOW color | ||||
| A. | Does not readily leave mark on paper but can be scratched by ordinary pocket knife | ||||
| Chalcopyrite | Greenish black | Brass-yellow or golden-yellow color that may tarnish and show rainbow-like colors; commonly massive; notice streak | 3½-4 | ||
| Gold | Shiny golden yellow | Shiny yellow color; extremely heavy; flattens when hit with hammer; notice streak | 2½-3 | ||
| B. | Cannot be scratched by ordinary pocket knife | ||||
| Pyrite | Black, greenish black, or brownish black | Shiny, pale golden-yellow or brass-yellow color that may tarnish; occurs as grains, as masses, or as cubes or other crystal shapes; notice hardness and streak | 6-6½ | ||
| 27 | |||||
| 4. | METALLIC or SUBMETALLIC luster, BROWN color | ||||
| A. | Does not readily leave mark on paper but can be scratched by ordinary pocket knife | ||||
| Limonite | Rusty yellowish brown | Dark-brown color; some specimens have a shiny black surface; notice streak | 5-5½ | ||
| B. | Cannot be scratched by an ordinary pocket knife | ||||
| Cassiterite | Pale brown, pale yellow or white | Brown; submetallic; heavy; notice streak | 6-7 | ||
| Hematite | Dark reddish brown | Dark brown color; commonly occurs as granular or compact masses; notice streak | 5½-6½ (may be softer) | ||
| Limonite | Rusty, yellowish brown | Dark brown color; some specimens have a shiny black surface; notice streak | 5-5½ | ||
| 5. | METALLIC or SUBMETALLIC luster, BLACK color | ||||
| A. | Will leave mark on paper | ||||
| Argentite | Shiny, blackish to lead grey | Lead-gray color that tarnishes to dull black; knife cuts it smoothly; heavy; may occur as masses and coatings | 2-2½ | ||
| Graphite | Black | Greasy feel; very soft; splits into thin flakes | 1-2 | ||
| Pyrolusite | Black | Very soft; will soil fingers; may be powdery | 1-2 | ||
| B. | Does not readily leave mark on paper but can be scratched by an ordinary pocket knife | ||||
| Chalcocite | Grayish black | Shiny lead-gray color that tarnishes to dull black; knife cuts it smoothly; may have a black sooty coating; commonly occurs as compact or granular masses | 2½-3 | ||
| Hollandite | Black | May occur as rounded masses | 4-6 | ||
| Limonite | Rusty, yellowish brown | Some specimens have shiny black surface; notice streak | 5-5½ | ||
| C. | Cannot be scratched by an ordinary pocket knife | ||||
| Braunite | Steel gray or black | Luster is submetallic | 6-6½ | ||
| Cassiterite | Pale brown, pale yellow, or white | Submetallic luster; heavy; notice streak | 6-7 | ||
| Hematite | Dark reddish brown | Notice streak; commonly occurs as granular or compact masses | 5½-6½ (may be softer) | ||
| 28 | |||||
| Hollandite | Black | May occur as rounded masses | 4-6 | ||
| Limonite | Rusty yellowish brown | Some specimens have shiny black surface; notice streak | 5-5½ | ||
| Magnetite | Black | Fragments cling to a magnet | 6 | ||
| Pitchblende | Brownish black | Brownish black, greenish black, or black; radioactive; heavy; may appear dull or greasy | 5½ | ||
| 6. | NONMETALLIC luster, WHITE color | ||||
| A. | Can be scratched by a fingernail | ||||
| Cerargyrite | Shiny white or gray | Appears waxy; knife cuts it smoothly; turns violet brown to black when exposed to light | 1-1½ | ||
| Gypsum | White | Soft; occurs as crystals or as fibrous, granular, compact, or earthy masses | 2 | ||
| Talc | White | Knife cuts it smoothly; feels soapy or greasy; splits into thin flakes | 1 | ||
| B. | Cannot be scratched by a fingernail but can be scratched by a copper penny | ||||
| Anhydrite | White | Commonly occurs as sugary-looking masses | 3-3½ | ||
| Barite | White | Rather heavy; cleavage fragments may be flat and slab-like | 3-3½ | ||
| Calcite | White | Dilute hydrochloric acid fizzes on calcite; perfect cleavage in 3 directions gives rhomb-shaped fragments | 3 | ||
| Celestite | White | Not quite as heavy as barite; crystals commonly prism-shaped or flat-looking; some cleavage fragments are flat and slab-like | 3-3½ | ||
| Halite | White | Salty taste; dissolves in water; cube-shaped cleavage fragments | 2½ | ||
| C. | Cannot be scratched by a copper penny but can be scratched by an ordinary pocket knife | ||||
| Anhydrite | White | Commonly occurs as sugary-looking masses | 3-3½ | ||
| Barite | White | Rather heavy; cleavage fragments may be flat and slab-like | 3-3½ | ||
| Celestite | White | Not quite as heavy as barite; crystals commonly prism-shaped or flat-looking; some cleavage fragments are flat and slab-like | 3-3½ | ||
| 29 | |||||
| Dolomite | White | Commonly occurs as granular masses and as rhomb-shaped crystals; dilute hydrochloric acid may fizz slightly on dolomite | 3½-4 | ||
| Fluorite | White | Cleavage in 4 directions can give fragments that are shaped like octahedrons; crystals commonly cubes | 4 | ||
| Opal | White | Curved, conchoidal fracture; may appear glassy, greasy, resinous, or dull; milky white and bluish-white precious opal shows plays of colors | 5-6 | ||
| D. | Cannot be scratched by an ordinary pocket knife but can be scratched by quartz | ||||
| Feldspar | White | Glassy or pearly luster; good cleavage in 2 directions that meet at an angle of 90° or near 90°; common in granite and pegmatite rocks | 6 | ||
| Opal | White | Curved, conchoidal fracture; may appear glassy, greasy, resinous, or dull; milky white and bluish-white precious opal shows plays of colors | 5-6 | ||
| Quartz | White | Curved conchoidal fracture; occurs as milky quartz, chert, and chalcedony; crystals commonly 6-sided prisms with pyramid-like ends | 7 | ||
| 7. | NONMETALLIC luster, GRAY color | ||||
| A. | Can be scratched by a fingernail | ||||
| Amphibole asbestos | White | Made up of slender, flexible fibers that can be pulled apart | 1-2½ | ||
| Cerargyrite | Shiny white or gray | Appears waxy; knife cuts it smoothly; turns violet brown to black when exposed to light | 1-1½ | ||
| Gypsum | White | Soft; occurs as crystals or as fibrous, granular, compact, or earthy masses | 2 | ||
| Sulfur | White or pale yellow | Will burn with a blue flame; commonly found as crystals, crusts, or grains | 1½-2½ | ||
| Talc | White | Knife cuts it smoothly; feels soapy or greasy; splits into thin flakes | 1 | ||
| B. | Cannot be scratched by a fingernail but can be scratched by a copper penny | ||||
| Amphibole asbestos | White | Made up of slender, flexible fibers that can be pulled apart | 1-2½ | ||
| Anhydrite | White | Commonly occurs as sugary-looking masses | 3-3½ | ||
| 30 | |||||
| Calcite | White | Dilute hydrochloric acid fizzes on calcite; perfect cleavage in 3 directions gives rhomb-shaped fragments | 3 | ||
| Celestite | White | Crystals commonly prism-shaped or flat-looking; some cleavage fragments are flat and slab-like | 3-3½ | ||
| Halite | White | Salty taste; dissolves in water; cube-shaped cleavage fragments | 2½ | ||
| Sulfur | White or pale yellow | Will burn with a blue flame; commonly found as crystals, crusts, or grains | 1½-2½ | ||
| C. | Cannot be scratched by a copper penny but can be scratched by an ordinary pocket knife | ||||
| Anhydrite | White | Commonly occurs as sugary-looking masses | 3-3½ | ||
| Celestite | White | Crystals commonly prism-shaped or flat-looking; some cleavage fragments are flat and slab-like | 3-3½ | ||
| Dolomite | White | Commonly occurs as granular masses and as rhomb-shaped crystals; dilute hydrochloric acid may fizz slightly on dolomite | 3½-4 | ||
| Opal | White | Curved, conchoidal fracture; may appear glassy, greasy, resinous, or dull | 5-6 | ||
| D. | Cannot be scratched by an ordinary pocket knife but can be scratched by quartz | ||||
| Feldspar | White | Glassy or pearly luster; good cleavage in 2 directions that meet at an angle of 90° or near 90°; common in granite and pegmatite rocks | 6 | ||
| Opal | White | Curved, conchoidal fracture; may appear glassy, greasy, resinous, or dull | 5-6 | ||
| Quartz | White | Curved conchoidal fracture; occurs as chert and chalcedony | 7 | ||
| 8. | NONMETALLIC luster, YELLOW color | ||||
| A. | Can be scratched by a fingernail | ||||
| Carnotite | Yellow | Bright canary yellow or lemon yellow; radioactive; occurs as crusts and powdery masses | 2 | ||
| Gypsum | White | Yellowish; soft; occurs as crystals or as fibrous, granular, compact or earthy masses | 2 | ||
| Limonite | Rusty yellowish brown | Brownish-yellow color; may be soft and earthy | 1+ | ||
| 31 | |||||
| Muscovite (white mica) | White | Light colored; splits into thin, flat, transparent sheets that will bend without breaking | 2-2½ | ||
| Sulfur | White or pale yellow | Will burn with a blue flame; commonly found as crystals, crusts, or grains | 1½-2½ | ||
| Uranophane | Light yellow to light yellow orange | Yellow to yellow-orange color; radioactive | 2-3 | ||
| B. | Cannot be scratched by a fingernail but can be scratched by a copper penny | ||||
| Barite | White | Rather heavy; cleavage fragments may be flat and slab-like | 3-3½ | ||
| Calcite | White | Yellowish; dilute hydrochloric acid fizzes on calcite; perfect cleavage in 3 directions gives rhomb-shaped fragments | 3 | ||
| Muscovite (white mica) | White | Light colored; splits into thin, flat, transparent sheets that will bend without breaking | 2-2½ | ||
| Sulfur | White or pale yellow | Will burn with a blue flame; commonly found as crystals, crusts, or grains | 1½-2½ | ||
| Uranophane | Light yellow to light yellow orange | Yellow to yellow-orange color; radioactive | 2-3 | ||
| C. | Cannot be scratched by a copper penny but can be scratched by an ordinary pocket knife | ||||
| Barite | White | Rather heavy; cleavage fragments may be flat and slab-like | 3-3½ | ||
| Opal | White | Curved, conchoidal fracture; may appear glassy, greasy, resinous, or dull | 5-6 | ||
| D. | Cannot be scratched by an ordinary pocket knife but can be scratched by quartz | ||||
| Feldspar | White | Glassy or pearly luster; good cleavage in 2 directions that meet at an angle of 90° or near 90° | 6 | ||
| Garnet | White | Commonly occurs as crystals | 6½-7 | ||
| Opal | White | Curved, conchoidal fracture; may appear glassy, greasy, resinous, or dull | 5-6 | ||
| Quartz | White | Curved conchoidal fracture; brownish-yellow smoky quartz crystals commonly 6-sided prisms with pyramid-like ends; chalcedony and jasper may be a shade of yellow, too | 7 | ||
| 32 | |||||
| 9. | NONMETALLIC luster, BROWN color | ||||
| A. | Can be scratched by a fingernail | ||||
| Gypsum | White | Brownish; soft; occurs as crystals or as fibrous, granular, compact or earthy masses | 2 | ||
| Limonite | Rusty yellowish brown | May be soft and earthy | 1+ | ||
| Muscovite (white mica) | White | Light colored; splits into thin, flat, transparent sheets that will bend without breaking | 2-2½ | ||
| Sulfur | White or pale yellow | Will burn with a blue flame; commonly found as crystals, crusts, or grains | 1½-2½ | ||
| B. | Cannot be scratched by a fingernail but can be scratched by a copper penny | ||||
| Barite | White | Rather heavy; cleavage fragments may be flat and slab-like | 3-3½ | ||
| Biotite (black mica) | White | Dark brown; splits into thin, flat sheets that will bend without breaking | 2½-3 | ||
| Calcite | White | Dilute hydrochloric acid fizzes on calcite; perfect cleavage in 3 directions gives rhomb-shaped fragments | 3 | ||
| Halite | White | Salty taste; dissolves in water; cube-shaped cleavage fragments | 2½ | ||
| Muscovite (white mica) | White | Light colored; splits into thin, flat, transparent sheets that will bend without breaking | 2-2½ | ||
| Sulfur | White or pale yellow | Will burn with a blue flame; commonly found as crystals, crusts, or grains | 1½-2½ | ||
| C. | Cannot be scratched by a copper penny but can be scratched by an ordinary pocket knife | ||||
| Barite | White | Rather heavy; cleavage fragments may be flat and slab-like | 3-3½ | ||
| Dolomite | White | Commonly occurs as granular masses and as rhomb-shaped crystals; dilute hydrochloric acid may fizz slightly on dolomite | 3½-4 | ||
| Fluorite | White | Cleavage in 4 directions can give fragments that are shaped like octahedrons; crystals commonly cube-shaped | 4 | ||
| D. | Cannot be scratched by an ordinary pocket knife but can be scratched by quartz | ||||
| Cassiterite | Pale brown, pale yellow, or white | Brown, reddish brown, or yellowish brown; heavy; dull to brilliant luster | 6-7 | ||
| 33 | |||||
| Feldspar | White | Glassy or pearly luster; good cleavage in 2 directions that meet at an angle of 90° or near 90°; common in granite and pegmatite rocks | 6 | ||
| Garnet | White | Commonly occurs as crystals | 6½-7 | ||
| Quartz | White | Curved conchoidal fracture; brown smoky quartz crystals commonly 6-sided prisms with pyramid-like ends; chalcedony, chert, and jasper may be a shade brown, too | 7 | ||
| Tourmaline | White | Dark brown variety is dravite; notice hardness, striations on crystals, and triangular cross section of some crystals | 7-7½ | ||
| E. | Cannot be scratched by quartz | ||||
| Tourmaline | White | Dark brown variety is dravite; notice hardness, striations on crystals, and triangular cross section of some crystals | 7-7½ | ||
| 10. | NONMETALLIC luster, BLACK color | ||||
| A. | Cannot be scratched by a fingernail but can be scratched by a copper penny | ||||
| Biotite (black mica) | White | Splits into thin, flat sheets that will bend without breaking | 2½-3 | ||
| B. | Cannot be scratched by an ordinary pocket knife but can be scratched by quartz | ||||
| Garnet | White | Commonly occurs as crystals | 6½-7 | ||
| Quartz | White | Curved conchoidal fracture; brownish-black smoky quartz crystals commonly 6-sided prisms with pyramid-like ends; chalcedony and chert may be black, too | 7 | ||
| Tourmaline | White | Black variety is schorl; notice hardness, striations on crystals, and triangular cross section of some crystals | 7-7½ | ||
| C. | Cannot be scratched by quartz | ||||
| Tourmaline | White | Black variety is schorl; notice hardness, striations on crystals, and triangular cross section of some crystals | 7-7½ | ||
| 11. | NONMETALLIC luster, GREEN color | ||||
| A. | Can be scratched by a fingernail | ||||
| Amphibole asbestos | White | Made up of slender, flexible fibers that can be pulled apart | 1-2½ | ||
| 34 | |||||
| Cerargyrite | Shiny white or gray | Light greenish color; appears waxy; knife cuts it smoothly; turns violet brown to black when exposed to light | 1-1½ | ||
| Muscovite (white mica) | White | Light colored; splits into thin, flat, transparent sheets that will bend without breaking | 2-2½ | ||
| Sulfur | White or pale yellow | Greenish; will burn with a blue flame; commonly found as crystals, crusts, or grains | 1½-2½ | ||
| Talc | White | Light greenish color; knife cuts it smoothly; feels soapy or greasy; splits into thin flakes | 1 | ||
| B. | Cannot be scratched by a fingernail but can be scratched by a copper penny | ||||
| Amphibole asbestos | White | Made up of slender, flexible fibers that can be pulled apart | 1-2½ | ||
| Biotite (black mica) | White | Dark green; splits into thin, flat, translucent sheets that will bend without breaking | 2½-3 | ||
| Calcite | White | Dilute hydrochloric acid fizzes on calcite; perfect cleavage in 3 directions gives rhomb-shaped fragments | 3 | ||
| Halite | White | Greenish tint; salty taste; dissolves in water; cube-shaped cleavage fragments | 2½ | ||
| Muscovite (white mica) | White | Light colored; splits into thin, flat, transparent sheets that will bend without breaking | 2-2½ | ||
| Serpentine | White | Two kinds: silky and fibrous, waxy and platy | 2½-4 | ||
| Sulfur | White or pale yellow | Greenish; will burn with a blue flame; commonly found as crystals, crusts, or grains | 1½-2½ | ||
| C. | Cannot be scratched by a copper penny but can be scratched by an ordinary pocket knife | ||||
| Fluorite | White | Cleavage in 4 directions can give fragments shaped like octahedrons; crystals commonly cubes | 4 | ||
| Malachite | Green | Bright green color; dilute hydrochloric acid will fizz on malachite | 3½-4 | ||
| Serpentine | White | Two kinds: silky and fibrous, waxy and platy | 2½-4 | ||
| D. | Cannot be scratched by an ordinary pocket knife but can be scratched by quartz | ||||
| Feldspar | White | Glassy or pearly luster; good cleavage in 2 directions that meet at an angle of 90° or near 90° | 6 | ||
| Garnet | White | Commonly occurs as crystals | 6½-7 | ||
| 35 | |||||
| 12. | NONMETALLIC luster, BLUE color | ||||
| A. | Cannot be scratched by a fingernail but can be scratched by a copper penny | ||||
| Anhydrite | White | Commonly occurs as sugary-looking masses | 3-3½ | ||
| Barite | White | Rather heavy; cleavage fragments may be flat and slab-like | 3-3½ | ||
| Calcite | White | Dilute hydrochloric acid fizzes on calcite; perfect cleavage in 3 directions gives rhomb-shaped fragments | 3 | ||
| Celestite | White | Not quite as heavy as barite; crystals commonly prism-shaped or flat-looking; some cleavage fragments are flat and slab-like | 3-3½ | ||
| Halite | White | Salty taste; dissolves in water; cube-shaped cleavage fragments | 2½ | ||
| B. | Cannot be scratched by a copper penny but can be scratched by an ordinary pocket knife | ||||
| Anhydrite | White | Commonly occurs as sugary-looking masses | 3-3½ | ||
| Azurite | Blue | Bright, intense blue color; dilute hydrochloric acid will fizz on azurite | 3½-4 | ||
| Barite | White | Rather heavy; cleavage fragments may be flat and slab-like | 3-3½ | ||
| Celestite | White | Not quite as heavy as barite; crystals commonly prism-shaped or flat-looking; some cleavage fragments are flat and slab-like | 3-3½ | ||
| Fluorite | White | Cleavage in 4 directions can give fragments that are shaped like octahedrons; crystals commonly cube-shaped | 4 | ||
| Opal | White | Curved, conchoidal fracture; may appear glassy, greasy, resinous, or dull; milky white and bluish-white precious opal shows plays of colors | 5-6 | ||
| C. | Cannot be scratched by an ordinary pocket knife but can be scratched by quartz | ||||
| Feldspar | White | Glassy or pearly luster; good cleavage in 2 directions that meet at an angle of 90° or near 90° | 6 | ||
| Opal | White | Curved, conchoidal fracture; may appear glassy, greasy, resinous, or dull; milky white and bluish-white precious opal shows plays of colors | 5-6 | ||
| 36 | |||||
| Quartz | White | Curved conchoidal fracture; occurs as crystalline quartz and as bluish chalcedony | 7 | ||
| D. | Cannot be scratched by quartz | ||||
| Topaz | White | Perfect basal cleavage gives flat, plate-like fragments; notice hardness | 8 | ||
| 13. | NONMETALLIC luster, RED or PINK color | ||||
| A. | Can be scratched by a fingernail | ||||
| Gypsum | White | Reddish; soft; occurs as crystals or as fibrous, granular, compact, or earthy masses | 2 | ||
| Hematite | Dark reddish brown | Brownish-red color; soft and earthy | 1+ | ||
| Sulfur | White or pale yellow | Reddish; will burn with blue flame; commonly found as crystals, crusts, or grains | 1½-2½ | ||
| B. | Cannot be scratched by a fingernail but can be scratched by a copper penny | ||||
| Anhydrite | White | Pinkish tint; commonly occurs as sugary-looking masses | 3-3½ | ||
| Barite | White | Pinkish tint; rather heavy; cleavage fragments may be flat and slab-like | 3-3½ | ||
| Calcite | White | Pink color; dilute hydrochloric acid fizzes on calcite; perfect cleavage in 3 directions gives rhomb-shaped fragments | 3 | ||
| Cinnabar | Dark red | Dark red or bright yellowish-red color; shiny, brilliant luster when pure; dull and earthy when impure; heavy | 2½ | ||
| Halite | White | Reddish tint; salty taste; dissolves in water; cube-shaped cleavage fragments | 2½ | ||
| Sulfur | White or pale yellow | Reddish; will burn with blue flame; commonly found as crystals, crusts, or grains | 1½-2½ | ||
| C. | Cannot be scratched by a copper penny but can be scratched by an ordinary pocket knife | ||||
| Anhydrite | White | Pinkish tint; commonly occurs as sugary-looking masses | 3-3½ | ||
| Barite | White | Pinkish tint; rather heavy; cleavage fragments may look flat and slab-like | 3-3½ | ||
| Dolomite | White | Pink color; commonly occurs as granular masses and as rhomb-shaped crystals; dilute hydrochloric acid may fizz slightly on dolomite | 3½-4 | ||
| 37 | |||||
| Fluorite | White | Pink color; cleavage in 4 directions can give fragments that are shaped like octahedrons; crystals commonly cubes | 4 | ||
| Opal | White | Reddish color; curved, conchoidal fracture; may appear glassy, greasy, resinous, or dull | 5-6 | ||
| D. | Cannot be scratched by an ordinary pocket knife but can be scratched by quartz | ||||
| Feldspar | White | Glassy or pearly luster; good cleavage in 2 directions that meet at an angle of 90° or near 90° | 6 | ||
| Garnet | White | Commonly occurs as crystals | 6½-7 | ||
| Opal | White | Reddish color; curved, conchoidal fracture; may appear glassy, greasy, resinous, or dull | 5-6 | ||
| Quartz | White | Curved, conchoidal fracture; occurs as rose quartz, as pink chert, and as agate and jasper | 7 | ||
| 14. | NONMETALLIC luster, PURPLE or VIOLET color | ||||
| A. | Cannot be scratched by a copper penny but can be scratched by an ordinary pocket knife | ||||
| Fluorite | White | Cleavage in 4 directions can give fragments that are shaped like octahedrons; crystals commonly cubes | 4 | ||
| B. | Cannot be scratched by an ordinary pocket knife but can be scratched by quartz | ||||
| Quartz, variety: amethyst | White | Curved, conchoidal fracture; amethyst crystals commonly 6-sided prisms with pyramid-like ends | 7 | ||
| 15. | NONMETALLIC luster, COLORLESS | ||||
| A. | Can be scratched by a fingernail | ||||
| Cerargyrite | Shiny white or gray | Appears waxy; knife cuts it smoothly; turns violet brown to black when exposed to light | 1-1½ | ||
| Gypsum | White | Transparent selenite variety commonly occurs as flat, diamond-shaped crystals; splits into thin, flat sheets that will not bend without breaking | 2 | ||
| Muscovite (white mica) | White | Splits into thin, flat, transparent sheets that will bend without breaking | 2-2½ | ||
| 38 | |||||
| B. | Cannot be scratched by a fingernail but can be scratched by a copper penny | ||||
| Barite | White | Rather heavy; cleavage fragments may be flat and slab-like | 3-3½ | ||
| Calcite | White | Dilute hydrochloric acid fizzes on calcite; perfect cleavage in 3 directions gives rhomb-shaped fragments | 3 | ||
| Celestite | White | Not quite as heavy as barite; crystals commonly prism-shaped or flat-looking; some cleavage fragments are flat and slab-like | 3-3½ | ||
| Halite | White | Salty taste; dissolves in water; cube-shaped cleavage fragments | 2½ | ||
| Muscovite (white mica) | White | Splits into thin, flat, transparent sheets that will bend without breaking | 2-2½ | ||
| C. | Cannot be scratched by a copper penny but can be scratched by an ordinary pocket knife | ||||
| Barite | White | Rather heavy; cleavage fragments may be flat and slab-like | 3-3½ | ||
| Celestite | White | Not quite as heavy as barite; crystals commonly prism-shaped or flat-looking; some cleavage fragments are flat and slab-like | 3-3½ | ||
| Dolomite | White | Commonly occurs as granular masses and as rhomb-shaped crystals; dilute hydrochloric acid may fizz slightly on dolomite | 3½-4 | ||
| Fluorite | White | Cleavage in 4 directions can give fragments that are shaped like octahedrons; crystals commonly cubes | 4 | ||
| Opal | White | Curved, conchoidal fracture; transparent hyalite variety resembles ice | 5-6 | ||
| D. | Cannot be scratched by an ordinary pocket knife but can be scratched by quartz | ||||
| Opal | White | Curved, conchoidal fracture; transparent hyalite variety resembles ice | 5-6 | ||
| Quartz | White | Curved, conchoidal fracture; rock crystal quartz commonly 6-sided prism with pyramid-like ends | 7 | ||
| E. | Cannot be scratched by quartz | ||||
| Topaz | White | Perfect basal cleavage gives flat, plate-like fragments; notice hardness | 8 | ||
In the rock identification charts on pages 40-41, the Texas rocks described in this book are arranged in four major groups according to their texture.
Consult Rock Chart 1, if the rock is glassy; Chart 2, if it is compact, dull, or stony; Chart 3, if it is granular; and Chart 4, if it is fragmental.
Two of the rock charts are subdivided. In Rock Chart 2, the compact, dull, or stony rocks are arranged according to hardness as follows:
In Rock Chart 3, the granular rocks also are arranged according to hardness into:
In the “remarks” column of the rock identification charts are included further tests that will aid you in identifying the rock.
For a more complete rock determination chart, you can consult a textbook, such as Rocks and Rock Minerals, by L. V. Pirsson and A. Knopf.
| Chart No. | Rock | Remarks | ||
|---|---|---|---|---|
| 1. | GLASSY appearance (rock is dark, smooth, and shiny) | |||
| Obsidian | Entire rock is glassy | |||
| Vitrophyre | Crystalline grains are scattered through the dark glassy mass | |||
| 2. | COMPACT, DULL, OR STONY appearance (individual grains too small to be recognized) | |||
| A. | Can be scratched by a fingernail | |||
| Chalk | Dilute hydrochloric acid fizzes on it | |||
| Clay | Earthy odor when breathed on | |||
| Diatomite | Crumbly | |||
| Rock gypsum | Made up of the mineral gypsum | |||
| Soapstone | Soapy or greasy feel | |||
| B. | Cannot be scratched by a fingernail but can be scratched by ordinary pocket knife | |||
| Dolomite | Dilute hydrochloric acid may fizz slightly on it; will fizz if rock is powdered | |||
| Limestone | Dilute hydrochloric acid fizzes on it | |||
| Serpentine rock | Commonly some shade of green | |||
| Shale | Breaks in flat, thin flakes; earthy odor | |||
| C. | Cannot be scratched by an ordinary pocket knife | |||
| Basalt | Dark colored and heavy | |||
| Chert | Hard, smooth, and porcelain-like | |||
| Rhyolite | Light to dark colored; may show streaks or flow structure | |||
| 3. | GRANULAR appearance (at least some of the individual grains are large enough to be seen without a magnifying glass) | |||
| A. | Can be scratched by an ordinary pocket knife | |||
| Limestone | Dilute hydrochloric acid will fizz on it | |||
| Marble | Dilute hydrochloric acid will fizz on calcite marble, and it may fizz slightly on dolomite marble | |||
| Rock gypsum | Made up of the mineral gypsum | |||
| Rock salt | Has a salty taste; made up of the mineral halite | |||
| 41 | ||||
| B. | Generally cannot be scratched by an ordinary pocket knife (some schist is softer) | |||
| 1. | Grains are of about equal size (equigranular) | |||
| Granite | Quartz and feldspar grains interlock | |||
| Pegmatite | Large interlocking grains; commonly feldspar, quartz, mica | |||
| Quartzite | Rock breaks across the quartz grains | |||
| Sandstone | Rock breaks through the cement but around the sand grains | |||
| 2. | Easily seen grains are scattered through a mass of finer grains | |||
| Basalt | Dark colored, heavy rock | |||
| Llanite | Rusty pink feldspar and blue quartz grains embedded in a brownish rock mass | |||
| Rhyolite porphyry | Light to dark colored rock; may show streaks or flow structure | |||
| 3. | Grains are arranged in layers | |||
| Gneiss | Interlocking grains are in straight or wavy bands | |||
| Schist | Splits in thin layers; some schist is soft enough to be scratched by a knife | |||
| 4. | FRAGMENTAL appearance (rocks are made up of fragments that are either loose or cemented together) | |||
| Breccia | Angular, gravel-size fragments that are cemented together | |||
| Conglomerate | Rounded, gravel-size fragments that are cemented together | |||
| Coquina | Shells and shell fragments that are cemented together | |||
| Gravel | Loose fragments | |||
| Pulverulent limestone | Loose, powdery fragments; dilute hydrochloric acid fizzes on them | |||
| Sand | Loose fragments no larger than a pinhead | |||
| Sandstone | Sand fragments that are cemented together | |||
| Volcanic ash | Loose, fine, gritty particles | |||
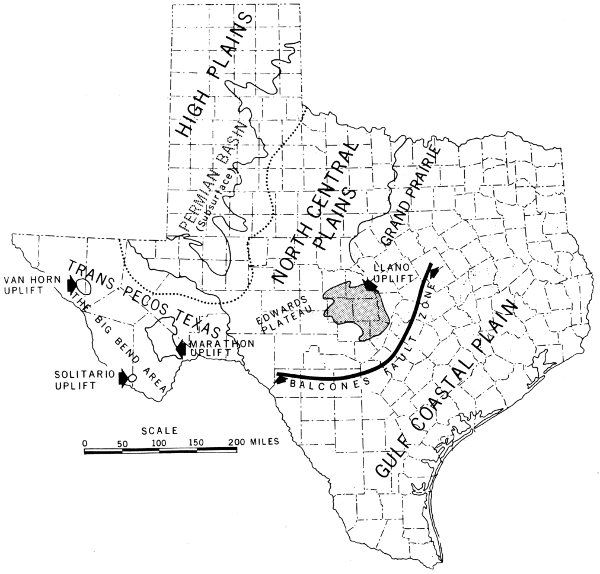
Physiographic outline map of Texas.
The pages that follow contain descriptions of Texas rocks and minerals. The descriptions are given in alphabetical order, except that related varieties are described together. For example, agate, amethyst, chert, flint, jasper, onyx, and chalcedony are discussed under quartz, because they are varieties of quartz. The descriptions include the properties of the rock or mineral that will help you identify it and also include information on where the rock or mineral can be found in Texas, some of its uses, and how it may have formed. The chart on page 99 lists chemical composition, specific gravity, and hardness of various Texas minerals.
Agate. See Quartz.
Agatized Wood. See Quartz.
Alabaster. See Gypsum.
Albite. See Feldspar.
Almandite. See Garnet.
Amethyst. See Quartz.
Amphibole Asbestos. See Asbestos.
Anhydrite, calcium sulfate, is a rather soft mineral that you can scratch with a pocket knife, although not with a fingernail. It has a glassy or a pearly luster and is transparent or translucent. Most anhydrite is white, but impurities cause it to be grayish, bluish, or reddish. When rubbed across a streak plate, anhydrite gives a white streak. This mineral has an uneven fracture, and it cleaves in three directions that are at right angles to each other. It commonly occurs as rectangular cleavage fragments or as sugary crystalline masses.
Anhydrite resembles dolomite, limestone, or gypsum. You can use a hardness test to distinguish it from gypsum (anhydrite is harder) and an acid test to distinguish it from limestone and dolomite. A drop of dilute hydrochloric acid will fizz when you put it on limestone or powdered dolomite. On anhydrite, the acid does not fizz.
Anhydrite occurs at several places in Texas. It is, for example, seen in bluffs along the Double Mountain Fork and the Salt Fork of the Brazos River in north-central Texas. Most of the Texas anhydrite, however, occurs underground. In the Gulf Coastal Plain, the anhydrite is found below the surface in salt domes. (Salt domes are described with halite on p. 66 and with sulfur on p. 91.)
Another anhydrite locality is in the subsurface Permian basin of west Texas. Oil wells drilled in this basin penetrate great, thick deposits of massive anhydrite. The anhydrite was deposited during the Permian Period from a sea that covered this area. As the sea gradually evaporated, the mineral matter that was dissolved in it came out of solution to form anhydrite, halite, and several other minerals.
Antigorite. See Serpentine.
Argentite. See Silver Minerals.
Asbestos is not really any one particular mineral. It is the name given to several 44 minerals that occur in masses of slender, delicate fibers. In the more typical kinds of asbestos, these fibers—when pulled apart—resemble soft, fluffy, silk strings.
Several small deposits of amphibole asbestos have been found in the Llano uplift area of central Texas. This asbestos is a variety of the mineral tremolite, a calcium-magnesium silicate. It has fibers that break rather easily, and it has a silky luster. It is a shade of green or gray and gives a white streak when rubbed across a streak plate. When you pull its fibers apart, you actually are breaking the mineral along its two directions of perfect cleavage. This amphibole asbestos is softer than other varieties of the mineral tremolite—a copper penny scratches it easily.

Greenish, silky amphibole asbestos from northeastern Gillespie County, Texas.
The asbestos occurs in veins in Precambrian metamorphic rocks in southern Llano County, northwestern Blanco County, and northeastern Gillespie County. These deposits are small.
A variety of the mineral serpentine called chrysotile asbestos is the kind most used by industry. Its fibers are commonly flexible enough and strong enough to be woven into cloth. This cloth is made into articles, such as fireproof suits, gloves, and theater curtains. Some chrysotile has been found in Precambrian metamorphic rocks in northwestern Blanco County, but it does not break into fibers fine enough or flexible enough to be called asbestos.
Azurite. See Copper Minerals.
Barite, barium sulfate, is a fairly common mineral in Texas. It has a glassy or a pearly luster, and it is transparent to translucent. Barite is colorless, white, brownish, bluish, yellowish, or reddish. When rubbed across a streak plate, it gives a white streak. It is not extremely hard—you can scratch it with a pocket knife, although not with a fingernail.
Barite is distinctive because of its weight and cleavage. It cleaves in three directions, and some cleavage fragments are flat or platy. For a mineral with a nonmetallic luster, barite is heavy—it has a specific gravity of 4.5.
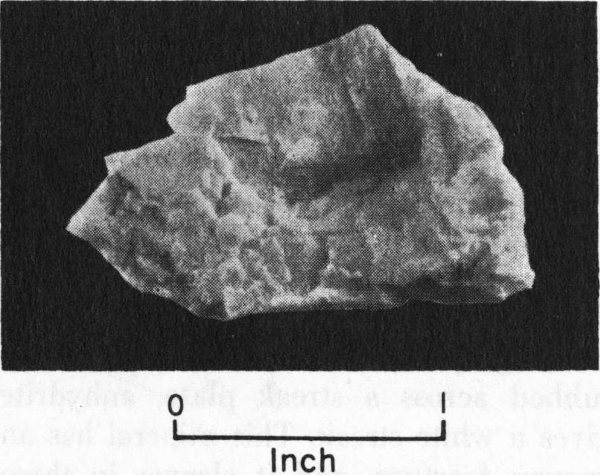
Barite cleavage fragment from west Texas.
Barite commonly occurs as prism-shaped and as flat crystals, as granular masses, as cleavable masses, and as rounded masses called nodules. In Texas, some of it was deposited in sedimentary rocks by underground waters. As the waters seeped through these rocks, mineral matter came 45 out of solution to form the barite. Some of the barite in Texas also formed from solutions that came from hot magmas.
A number of barite deposits have been found in Texas, but many of them are small. Barite occurs in Precambrian metamorphic rocks in Gillespie and Llano counties, in Pennsylvanian shale in Brewster County, in Permian shales in Baylor and Taylor counties, and in Permian limestones in Culberson County. It is found in Triassic red shales in Howard County and in Cretaceous sedimentary rocks in Brewster, Brown, Hudspeth, Jeff Davis, Kinney, and Val Verde counties. In Live Oak County, barite occurs in Tertiary bentonitic clays. Barite is being mined from a deposit in the Seven Heart Gap area northeast of Van Horn in Culberson County.
Barite is used in a number of ways. It is a source of barium chemicals, and it also is powdered and used as an ingredient in paint. The oil industry uses large amounts of barite. In drilling for oil by the rotary method, water and muds are pumped down the hole to aid drilling. Barite is added to these drilling fluids to make them heavy, since high-pressure gases are not as likely to blow heavy fluids out of the hole.
Basalt is a heavy igneous rock that is black, dark gray, or dark brown. This rock is made up chiefly of a feldspar mineral, such as labradorite, and a pyroxene mineral, such as augite. Other minerals may be present.
The mineral grains of some basalts are so small that you cannot distinguish them even with a magnifying glass. Other basalts, however, are porphyritic, which means that they contain larger, easily seen crystals and grains of feldspar and pyroxene scattered either through a mass of the small mineral grains or through glassy material.
Some basalts contain many small holes. These holes, called vesicles, were formed when bubbles of gas were trapped in the hardening magma. Later, solutions moving through the rocks may have deposited another mineral—such as calcite or chalcedony—in some of the vesicles.
Basalt forms from molten rock material that hardens either on or beneath the surface—it can be extrusive or intrusive. Much of the basalt now found in the Trans-Pecos country of west Texas formed from lava that flowed out onto the surface during the Tertiary Period. A few of the places where basalt occurs in west Texas are the Chinati Mountains of Presidio County, the Chisos Mountains of Brewster County, the Davis Mountains of Jeff Davis County, and the Van Horn Mountains of Culberson and Hudspeth counties.

Basalt from Brewster County, Texas.
Several varieties of basalt occur in the Balcones fault region of Bandera, Comal, Hays, Kinney, Medina, Travis, and Uvalde counties. These basalts formed from molten magma that forced its way into rocks just below the earth’s surface.
Some basalt, which is known commercially as trap rock, is produced in Uvalde County. It is crushed and used for railroad ballast, road building material, and as concrete aggregate.
Bentonite. See Clay.
Biotite. See Mica.
Braunite. See Manganese Minerals.
Calcite, calcium carbonate, is one of the most abundant minerals in Texas. It is the chief mineral in limestone and in some marble. It also serves as the cementing material in many sandstones. Crystals, grains, and cleavable masses of calcite, which have been deposited by underground water, occur in cracks and cavities in many of the igneous, metamorphic, and sedimentary rocks of Texas. Calcite also occurs as cave, spring, and stream deposits and as caliche.
Calcite is transparent or translucent, and—depending on the variety—its luster is glassy to earthy. Most calcite is white or colorless, but it can be a shade of pink, blue, green, brown, yellow, or gray. It gives a white streak when you rub it across a streak plate.
Two properties of calcite to notice are the hardness and the cleavage. This mineral cleaves perfectly in three directions that are not at right angles to each other, and some of the cleavage fragments are rhombohedrons. Calcite is rather soft—you can scratch it with a copper penny but not with a fingernail. A drop or two of dilute hydrochloric acid also will help you to identify this mineral. The acid will readily fizz and bubble when it is placed on calcite.
Calcite has perfect rhombohedral cleavage. The three directions of cleavage are not at right angles to each other.
Calcite occurs in more different kinds of crystal shapes than any other mineral. Some of these crystals are flat and tabular; some are rhombohedrons; some are prisms. Pointed crystals, called dog-tooth spar, and twinned crystals have been found in the Terlingua area of Brewster County in west Texas. A somewhat unusual occurrence of calcite crystals is in geodes. Some of these are found in Lower Cretaceous rocks west of Austin in Travis County.
Transparent crystals and cleavage fragments of calcite show a property called double refraction (other minerals show it, too). To test this property, you can mark a single dot on paper. When you look at the dot through a piece of clear calcite, you will see two dots instead of one. This happens because a ray of light is bent (refracted) and is split into two rays as it enters the mineral. These two rays travel through the calcite in slightly different directions, and each carries an image of the dot through the mineral. The two images that you see are at the points where the two rays leave the calcite.
Calcite that is deposited at springs, along river and creek banks, and in caves is known as travertine. Cave forms of travertine, including stalagmites and stalactites, occur in several caves in Texas. Another kind of travertine is called calcareous tufa or calcareous sinter. It is a porous and spongy-looking material deposited from water carrying dissolved limestone and is found around the openings of some springs and along some creek and river banks.
A dull, earthy calcite deposit, known as caliche, occurs in areas of Texas that have scant rainfall, such as the High Plains, west Texas, and the southwestern part of the Gulf Coastal Plain. Caliche commonly is found mixed with other materials, such as clay, sand, or gravel. This substance may be firm and compact or loose and powdery.
It is thought that caliche forms when ground moisture, containing dissolved calcium bicarbonate, moves upward. In dry 47 areas of the country, this moisture evaporates. As it does, it leaves a crust of calcium carbonate in the form of caliche on or near the surface of the ground.

Calcite crystals (dog-tooth spar) from the Terlingua area of Brewster County, Texas.
Caliche is quarried in many counties in Texas and is used chiefly as road material and as an aggregate.
Caliche. See Calcite.
Carnotite. See Uranium Minerals.
Cassiterite, tin dioxide, is the mineral that serves as the chief source of tin. Tin does not corrode and tarnish, and one of its main uses is in the making of tin cans. (Actually, our tin cans are made from thin sheets of steel that have been coated with a protective layer of tin.)
Cassiterite has either a nonmetallic or a submetallic luster. Some specimens are brilliant and shiny; others are dull. Cassiterite may be translucent to transparent. It may be black, brown, gray, reddish brown, or yellowish brown. When rubbed across a streak plate, this mineral leaves a pale brown, a pale yellow, or a white streak. Cassiterite is quite heavy—it has a specific gravity of 6.8 to 7.1. It is too hard to be scratched by an average pocket knife.
Sometimes, prospectors use a chemical test to help them identify cassiterite. They put small pieces of metallic zinc into a jar or test tube containing dilute hydrochloric acid. Then they add a few fragments of the mineral that they suspect is cassiterite. If the fragments are cassiterite, they become covered with a pale gray coating of metallic tin.
Cassiterite’s most common crystal shape is a short, 8-sided prism with pyramids at each end, but perfect crystals are not often found. Most Texas cassiterite does not show a crystal shape. Instead, it occurs as crystalline masses in igneous rocks and as loose pebbles that have weathered out of these rocks.
Cassiterite occurs in a number of places in the United States but not in large quantities. A small amount of cassiterite has been found in quartz veins in Precambrian granite in both central Texas and west Texas. In El Paso County, the cassiterite is found on the east side of the Franklin Mountains a few miles north of El Paso, where some of it has been mined. In central Texas, cassiterite occurs in the Streeter area of Mason County.
When the granite rocks in these areas were formed, probably not all of the hot magmas cooled and hardened at the same time. The fluids given off by the remaining magmas contained tin and several other elements. It is believed that these fluids moved up into cracks in the granite rocks and formed the cassiterite.
Celestite is a strontium sulfate mineral. It is colorless, white, yellow, or gray. Light blue specimens of this mineral also are found, and it is because of this sky-like color that celestite gets its name. The word celestite comes from the Latin word caelestis, meaning of the sky.
Celestite has a glassy to a pearly luster, and it is either transparent or translucent. It gives a white streak when rubbed across a streak plate. Celestite has a specific gravity of 3.95 to 3.97. It is, however, lighter than barite, a mineral that it resembles. Celestite is not very hard—a knife will scratch it, although your fingernail will not. It cleaves in three directions, and some of the fragments are flat and slabby.
Celestite cleavage fragment from Lampasas County, Texas.
Celestite occurs commonly either as 49 prism-shaped or flat crystals and as cleavable, granular, or fibrous crystalline masses. In Texas, it is found in geodes, as rounded nodules, or as bedded or layer-like deposits in limestones and other sedimentary rocks. In Real County, celestite occurs on the walls of a cave in Cretaceous limestone.
Some celestite may be deposited by sea water, but much of the Texas celestite is believed to have been deposited by underground water that seeped through cracks and pores in the limestones and other sedimentary rocks. This water picked up and dissolved strontium compounds that were scattered in small amounts through the rocks. Then, it re-deposited the strontium in the rocks as celestite.
In Texas, beds of celestite occur in Permian rocks in Coke, Fisher, and Nolan counties and in Lower Cretaceous rocks in Brown, Comanche, and Mills counties. Celestite geodes and nodules are found in Lower Cretaceous limestone rocks in Lampasas, Travis, and Williamson counties, and in Permian rocks in Coke, Fisher, Nolan, and Taylor counties.
Celestite is one of two minerals (the other mineral is strontianite, strontium carbonate) used as a source of strontium. Strontium compounds give a crimson-red color to a flame, so they are used in fireworks, tracer bullets, and flares. Perhaps you have seen a red flare set out on the highway at night to warn motorists that a truck has stalled. The chances are good that the flare’s red flame was due to a strontium compound. Some of the Texas celestite has been mined, but most of the strontium minerals now used in the United States are imported from England and Mexico.
Cerargyrite. See Silver Minerals.
Chalcedony. See Quartz.
Chalcocite. See Copper Minerals.
Chalcopyrite. See Copper Minerals.
Chalk. See Limestone.
Chrysotile. See Asbestos; Serpentine.
Cinnabar, which is mercuric sulfide, is the most common mercury mineral. It has a dark red or a bright yellowish-red color and is transparent to translucent. When rubbed across a streak plate, it leaves a dark red streak. If pure, cinnabar has a brilliant, shiny, nonmetallic luster. It is, however, commonly found mixed with impurities, such as clay, calcite, iron oxide, or bituminous material, and then it looks dull and earthy. Cinnabar is quite heavy—it has a specific gravity of 8.10. It is rather soft, and you can scratch it with a copper penny.
Some prospectors use a quick chemical test to identify cinnabar. They rub a clean, shiny copper coin with a mineral sample that has been moistened with a drop or two of dilute hydrochloric acid. If the sample is cinnabar, a light silvery-gray coating appears on the coin.
Cinnabar occurs as small crystals or as fine-grained or compact crystalline masses. It is found in veins that fill cracks in rocks and also occurs as crusts and coatings on rocks. It also may be widely scattered through rocks, such as limestones.
Cinnabar occurs in the Terlingua area of Brewster and Presidio counties in west Texas. It has been mined there, off and on, since about 1894, and during this time, mercury worth many millions of dollars has been produced.
Most of this west Texas cinnabar is found in cracks, pores, and breccia-filled cavities of Cretaceous limestones and clays. If you will look at the Texas geologic map (pp. 4-5), you will see that igneous rocks occur in this district. Many millions of years ago during the Tertiary Period, when these igneous rocks were still hot magma, some of them pushed up under the Cretaceous rocks and emitted fluids containing mercury. The fluids moved upward through cracks and pores in the Cretaceous 50 rocks where they deposited the mercury as cinnabar and as other mercury minerals.

Cinnabar crystals (dark) with calcite crystals (white) from the Terlingua area of Brewster County, Texas.
Mercury is an unusual element. Instead of occurring as a solid metal at ordinary room temperatures, as do gold, silver, and lead, it remains a liquid until it is cooled to 38 degrees below zero Fahrenheit. Because the silvery little drops of liquid mercury roll about as if they were alive, this element long has been called quicksilver.
Mercury is used in a variety of ways. In some noiseless light-switches, a glass tube containing a small ball of mercury tilts when the switch is turned “on.” The mercury then rolls to the end of the tube that contains electrical contacts and quietly completes the electrical circuit. In other uses, mercury is added to silver, tin, and other metals to make fillings for teeth. Some medicines, such as calomel and mercurochrome, contain mercury. Fulminate of mercury helps to set off dynamite and other explosives. Mercury is used in many barometers and thermometers, and farmers use mercury poisons to control insects and fungi.
Mercury also commonly is used to obtain gold from its ores. One method of accomplishing this is to pass wet gold-bearing gravel or crushed rock over metal plates that are coated with mercury. The gold particles quickly mix with the mercury to form an amalgam, which later can be scraped off the plates. The gold is then recovered by heating the amalgam to drive off the mercury.
Clay is a smooth, soft, earthy rock made up of mineral particles no bigger than specks of dust. Some of the particles are clay minerals, which consist of aluminum, silicon, and other elements. In addition, tiny particles of quartz, calcite, and other minerals may also be present in the clay.
The clay particles are all that remain of rocks and of minerals, such as feldspar, that have been broken into fragments or altered into clay minerals by weathering. Some clay remains at the place where it formed, but some is carried away and deposited elsewhere.
Clay is white, tan, brown, red, green, blue, gray—almost any color. When moist, it has an earthy odor. You can moisten a piece of clay enough to notice this just by breathing on it. Most clays, when wet, can be molded into many different shapes—that is, they are plastic, but when they are dry, they are firm and solid.
Clay is abundant in Texas and has a number of uses. Some goes to make portland cement, and some is baked or burned in a kiln to make brick, tile, sewer pipes, pottery, and other products. This kind of clay is obtained from Tertiary formations of the Gulf Coastal Plain, from Upper Cretaceous formations in central Texas, and from Pennsylvanian formations in north-central Texas. (You can locate Tertiary, Cretaceous, and Pennsylvanian rocks on the Texas geologic map, pp. 4-5.)
A special kind of white burning clay that can be used to make chinaware is called kaolin or china clay. It contains particles of the clay mineral kaolinite as well as several other clay minerals. Deposits of china clay occur in southern Jeff Davis County and in Real County near Leakey, but none is being produced.
Another kind of clay, bentonite, forms from weathered volcanic ash. Bentonite contains the clay mineral montmorillonite and looks smooth and soap-like. Fresh samples of this clay are white, pale green, or pale blue, but dried-out or weathered samples are tan, brown, yellow, or reddish. When wet, bentonite absorbs water, swells, and then has a jelly-like appearance.
Surface deposits of bentonite occur chiefly in Eocene Tertiary formations of the Gulf Coastal Plain, in Cretaceous formations of the Big Bend area of west Texas, and in Quaternary formations of the High Plains.

Bentonite is used as a drilling-fluid additive in the rotary method of drilling for petroleum and gas.
Some bentonite is used to absorb unwanted coloring material in petroleum and in vegetable oils. It is then known as a bleaching clay. Bentonite bleaching clay is obtained from some of the Tertiary formations along the Texas Gulf Coastal Plain. 52 It has been produced in Angelina, Fayette, Gonzales, Jasper, Walker, and other counties in this area.
Another important use of bentonite, and of other clay, too, is as drilling mud. In the rotary method of drilling for oil and gas, mud is pumped down into the drilled hole. This mud carries the rock cuttings up to the surface, it cools the drilling tools, and it coats and seals the walls of the hole. Along the Gulf Coastal Plain, drilling clay is obtained from Tertiary formations.
Common Opal. See Opal.
A number of minerals containing copper, such as chalcocite, chalcopyrite, malachite, and azurite, occur in small deposits in Texas. They are found chiefly in the Llano uplift area of central Texas, in the Van Horn area of Culberson and Hudspeth counties in west Texas, and in a group of counties in north-central Texas.
Copper is an important element. Because it is an unusually good conductor of electricity (only silver, which costs much more, is a better one), it is used for many kinds of wires for switchboards, generators, motors, telephone and telegraph equipment, and light and power lines.
Manufacturers commonly combine copper with other elements. For example, some copper is mixed with zinc to make brass and with tin and a little zinc to make bronze. These mixtures are called alloys. Many products are made from copper alloys, including tubing, pipes, jewelry, pots, and pans. Even our coins contain copper.
Sometimes, a prospector uses a chemical test to find out if copper is present in a mineral. First, he crushes a small sample of what he believes is a copper mineral (such as chalcocite, chalcopyrite, azurite, or malachite). He then puts the sample in a glass jar or test tube and pours in a small amount of dilute nitric acid (this acid, like hydrochloric acid, is poisonous). After the sample has dissolved in the acid, he adds enough ammonium hydroxide to make the solution alkaline. If the sample is a copper mineral, the solution turns a deep-blue color.
One of the copper minerals, chalcocite, copper sulfide, also is known as copper glance. It is a metallic mineral that commonly tarnishes to a dull black. By chipping off a fragment to obtain a fresh surface, you will see that it has a shiny lead-gray color. Chalcocite is rather soft, and it is sectile, that is, a knife will cut through it as well as scratch it. When you rub chalcocite across a streak plate, it gives a grayish-black streak. This mineral commonly occurs as compact masses or as granular masses.
Chalcocite, with its dark color, does not look at all like copper, which is a bright reddish brown. Chalcocite, however, is the chief copper mineral at the most important copper mine in Texas, the Hazel mine, which is about 15 miles northwest of Van Horn in Culberson County in west Texas. This mine, although now idle and almost filled with water, has produced about one and a half million pounds of copper along with more valuable silver ores. Here, the chalcocite and other minerals occur in material that fills large cracks in red sandstone of the Precambrian Hazel Formation. It is thought that long ago, molten igneous rock material far below the surface sent out hot solutions containing copper and other elements. These solutions moved upward and deposited minerals in the fracture zone in the sandstone.
Chalcocite occurs also in north-central Texas. It is found in Archer, Baylor, Clay, Foard, Hardeman, King, Knox, Stonewall, and several other counties of this area. Here, it occurs in Permian sedimentary rocks (called “red beds”) as rounded masses, as scattered grains, and as petrified wood. Because these deposits are far from any igneous rocks, they apparently did not form in the same way as those at the Hazel mine. These north-central Texas deposits have never really been commercially developed. During the Civil War, however, some copper from this area was made into percussion caps for the Confederacy.
The Hazel copper-silver mine, Culberson County, Texas, as it appeared in 1951. Photograph by P. T. Flawn.
Another copper mineral, chalcopyrite, is a copper-iron sulfide. It also is known as copper pyrites and yellow copper ore. This mineral has a metallic luster and a brass-yellow or a golden-yellow color. When rubbed across a streak plate, it gives a greenish-black streak. Chalcopyrite will tarnish and then has bronze, blue, purple, and other rainbow-like colors. This mineral is fairly soft—you can scratch it with a pocket knife. Because of chalcopyrite’s yellow color, it has often been mistaken for gold. For this reason, it, like iron pyrite, is often called fool’s gold. (See Gold, p. 60, for ways to tell them apart.)
Chalcopyrite commonly is found in compact masses that show no crystal shapes. These masses either are scattered through rocks or occur in material that fills cracks in rocks.
Some chalcopyrite is found in Precambrian sandstone at the Hazel mine and in other deposits in the Van Horn area of Culberson and Hudspeth counties. It also occurs in Precambrian rocks at the Sheridan and Pavitte prospects in Burnet County. These chalcopyrite localities are in districts where igneous rocks occur.
It is likely that, long ago, hot solutions containing copper moved upward, out of deeply buried molten magma. While still far below the surface, the solutions deposited the chalcopyrite in cracks and other openings in the nearby rocks.
Two copper minerals of Texas, azurite and malachite, are copper carbonates. Azurite is commonly called chessylite and blue copper; malachite is called green copper carbonate. Because these minerals are carbonates, a drop of dilute hydrochloric acid will fizz and bubble when placed on either of them.
Azurite has a bright, intense blue color and leaves a blue streak when rubbed across a streak plate. Malachite has a bright green color and leaves a green streak. These minerals have a nonmetallic luster and a glassy to dull appearance. Commonly, they are translucent, although some specimens of azurite are transparent. Both azurite and malachite are fairly soft—a pocket knife will scratch them, but a copper penny will not.
Azurite and malachite occur as individual 54 crystals, but you are more likely to find them as crusts on rocks and on other minerals. Malachite is also found in rounded fibrous masses that resemble bunches of grapes (described then as botryoidal).
Both azurite and malachite are formed in the same way. Underground waters seep through rocks that contain deposits of copper minerals (such as chalcocite and chalcopyrite) and cause chemical reactions which change these minerals into malachite and azurite.
Malachite is more plentiful than azurite, but both minerals can be found together. You can expect to find at least one of them at the same localities where chalcocite, chalcopyrite, and other copper minerals occur.
Coquina. See Limestone.
Diatomite. See Opal.
Dolomite is the name given both to a rock and to a mineral. The mineral is a calcium-magnesium carbonate and has a glassy or a pearly luster. It is any of a number of colors, such as white, pink, brown, or gray, or it can be colorless. Dolomite leaves a white streak on a streak plate and is transparent to translucent. It is not particularly hard and can be scratched with a pocket knife, although not with a copper penny. Dolomite cleaves perfectly in three directions, and some of the cleavage fragments are rhombohedrons. However, the cleavages of the individual mineral grains in specimens of fine-grained massive dolomite are not readily distinguishable.

Dolomite rock from the vicinity of Fairland, Burnet County, Texas.
Most Texas dolomite occurs as coarse-, medium-, and fine-grained crystalline masses as the chief mineral in dolomite rock and in dolomitic marble. It is also found as 6-sided crystals that are rhomb-shaped; when the faces are curved, they have a saddle-like appearance.
Crystals of the mineral dolomite commonly occur in cavities in the dolomite rocks. It is believed that they were deposited there by seeping underground waters. The waters dissolved some of the dolomite in the rocks and then re-deposited it as crystals.
Dolomite rock is made up mostly of crystalline grains of the mineral dolomite. In addition, quartz grains, calcite, and other minerals may be present. Dolomite rock is almost any color—white, buff, pink brown, gray. It resembles some limestone, and these two rocks actually are closely related.
To help tell them apart, dilute hydrochloric acid often is used. A few drops of this acid will readily fizz and bubble if the rock you put them on is a limestone. If the rock is dolomite, the acid will effervesce only very little or not at all. (If, however, the acid is put on powdered dolomite, it then will fizz readily.) Dolomite is slightly harder than limestone, and it also is slightly heavier.
Some dolomite rocks formed directly from materials that were dissolved in sea water, and others are altered limestone rocks. Some limestones altered into dolomite on the sea floor by the addition of magnesium from the sea water. Others changed into dolomite much later after the sea had withdrawn and the limestones had become a part of the land; underground waters containing magnesium seeped 55 through these limestones and altered them into dolomite.
Many of the dolomite rocks are found with limestones. In Texas they occur mostly in Cambrian, Ordovician, Mississippian, Pennsylvanian, Permian, and Cretaceous formations. The geologic map (pp. 4-5) indicates where these strata appear at the surface in Texas.
Dolomite is abundant in the Llano uplift area of central Texas—particularly in the Cambrian and Ordovician rocks. A number of these central Texas dolomites have been quarried for use as building stones. Some of them also have been crushed and used as a road-building material and as a stone aggregate that is mixed with cement to make concrete. This dolomite is also used as terrazzo chips (terrazzo floors are described with serpentine on p. 88). In addition, Ellenburger (Ordovician) dolomite from Burnet County was used during World War II as a source of the lightweight metal magnesium.
Dravite. See Tourmaline.
Feldspar is the name given to a group of nonmetallic minerals that are much alike. Several of them are so similar that a petrographic microscope must be used to tell them apart. Each of the feldspar minerals is an aluminum silicate. Each of them contains, in addition, at least one of the following elements: potassium, sodium, calcium, and barium. The feldspar minerals that are found in Texas include albite, a sodium-aluminum silicate, and orthoclase and microcline, which are both potassium-aluminum silicates.
The feldspar minerals are transparent to translucent and have either glassy or pearly lusters. They can be white, cream, or a shade of red, brown, yellow, blue, gray, or green. When you rub a feldspar across a streak plate, it leaves a white streak. The feldspars are rather hard—a pocket knife will not scratch them, although a piece of quartz or a steel file will. These minerals have good cleavage in two directions. The cleavages meet at an angle of about 90°, so that the cleavage fragments have square corners.

Feldspar cleavage fragment from Llano County, Texas. The two directions of good cleavage meet at an angle of about 90°.
The feldspars are important rock-forming minerals. You can find them in igneous rocks, such as granite or pegmatite, and in metamorphic rocks, such as gneiss. They also occur as fragments in sedimentary rocks, such as some sandstone and conglomerate.
Although the feldspars can originate in other ways, they form mostly from hot magmas that cool and crystallize into igneous rocks. These minerals occur in the rocks as grains, as cleavable masses, and as individual crystals. The crystals may be shaped like prisms, or they may be flat and slabby.
Good places to look for feldspars are in areas where granites, pegmatites, and other intrusive igneous rocks appear at the surface. The pegmatite rocks of Burnet, Gillespie, Llano, and Mason counties in the Llano uplift area of central Texas, and those of the Van Horn Mountains in Hudspeth and Culberson counties in west Texas, are especially good sources of feldspar. Large cleavable masses and crystals that are more than a foot long are found in some of these rocks.
The feldspars have a number of uses. Some of the pegmatite feldspars from Llano County in central Texas have been crushed 56 and used as granules for built-up and composition roofs. In addition, some have been shipped to Mexico for glass-making. Some of the other uses of feldspar are in making porcelain, ceramic glazes, and scouring compounds. A few of the feldspar minerals, such as the variety of microcline known as amazonstone, are used as gemstones.

Microcline feldspar crystals from near Granite Shoals Lake, Llano County, Texas.
Fibrous Gypsum. See Gypsum.
Flint. See Quartz.
Fluorite is calcium fluoride. The fluorite that is mined and sent to market, however, commonly is found mixed with quartz, calcite, limestone, or other rocks and minerals. Industry calls this mixture fluorspar.
Fluorite is a transparent to translucent mineral that has a glassy luster. It may be colorless, or it may be white, pink, green, purple, brown, or blue. Some specimens show more than one color. When you rub fluorite across a streak plate, it leaves a white streak. This mineral is not particularly hard—a pocket knife will scratch it, although a copper penny will not. Fluorite has perfect cleavage in four directions. By carefully breaking a specimen, you can obtain cleavage fragments that are shaped like octahedrons.
Fluorite occurs as cleavable masses, as fine or coarse grains, and as crystals. Most of the crystals are cubes, but some may be octahedrons, dodecahedrons, or combinations of these.
Fluorite has been found both in west Texas and in central Texas. In the Llano uplift area of central Texas, it occurs in a number of Precambrian granite, pegmatite, schist, and gneiss rocks. The most important, although small, deposit in this area is near Spring Creek a few miles west of Burnet in Burnet County. Here, prospectors have dug holes and pits in gneiss and schist rocks and found layers of fluorite in them.
The largest known fluorite deposits in Texas (they are not particularly large when you compare them with the deposits in Illinois and Kentucky) are those in the Eagle Mountains of Hudspeth County. This fluorite occurs in both igneous and sedimentary rocks. Many years ago, probably during the late part of the Tertiary Period, hot magma far below the surface gave off liquids and gases containing fluorine. These fluids moved up through large cracks 57 (called faults) in Cretaceous limestones and Tertiary igneous rocks and deposited fluorite in them. In places, beds of limestone have been replaced by fluorite. Some of this west Texas fluorite has been mined and shipped to market.
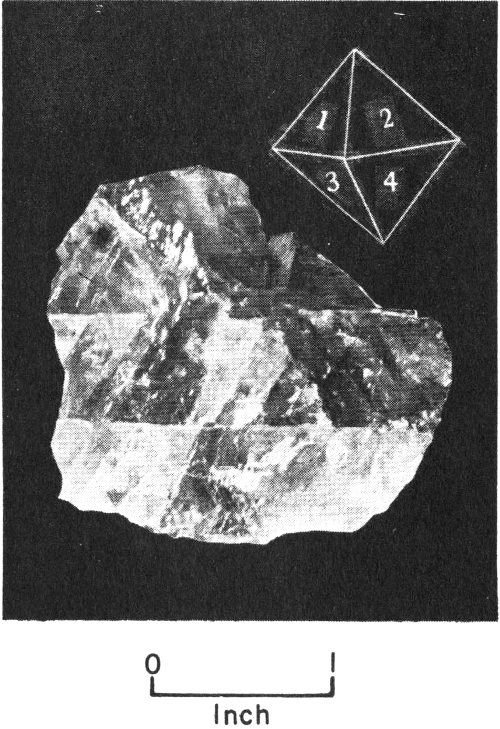
Fluorite has octahedral cleavage. The four directions of perfect cleavage can result in cleavage fragments that are octahedrons.
Fluorite is extremely important as a flux in steel-making to help the ingredients of the molten steel blend together. In addition, it combines with sulfur, phosphorus, and other unwanted substances so that they can be removed from the steel. Other important uses of fluorite are in glass-making and in the manufacture of hydrofluoric acid. This acid is used in the aluminum industry as well as in industries that make high-octane gasoline, insecticides, and refrigerants for refrigerators and freezers.
Galena, lead sulfide, is a shiny, lead-gray, metallic mineral that has a specific gravity of 7.4 to 7.6. It is soft enough to mark paper, and it leaves a grayish-black streak on a streak plate. This mineral cleaves perfectly in three directions, and the cleavage fragments have square corners—some are cubes.
Galena occurs as cleavable masses, as fine or coarse grains, and as crystals, most of which are cubes. Galena commonly is associated with other minerals; for example, some of the west Texas galena either contains some silver (then called argentiferous galena) or occurs with it. Sphalerite, a zinc mineral, is commonly found with galena.
Galena is an important mineral because it is the chief source of lead. Compounds of lead, called white lead, red lead, and litharge, are used as paint pigments. Automobile batteries contain lead plates, and tetraethyl lead is added to gasoline to keep the car’s motor from knocking. Some other uses of lead are in bullets, type metal, solder, and cable coverings.
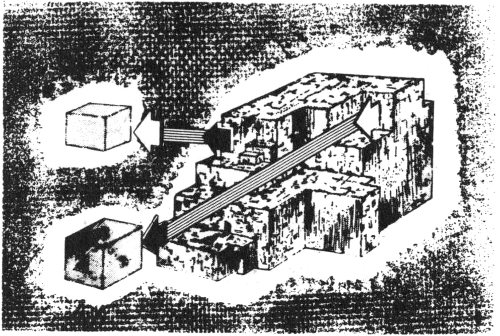
Galena has perfect cubic cleavage. The three directions of cleavage are at right angles to each other resulting in cubic cleavage fragments.
Galena has been found in several areas of Texas and has been mined in central and west Texas. None, however, has been produced in recent years. Most of the galena mined in west Texas was obtained from silver mines, where the galena was a by-product. Some of the west Texas galena deposits are at Altuda Mountain east of Alpine in Brewster County, in the Eagle Mountains and the Quitman Mountains in Hudspeth County, and in the Chinati Mountains and the Shafter area in Presidio County. Most of the mining has been 58 from the Shafter area (this area is described with silver minerals on p. 90).
In central Texas, several small galena deposits have been found in Blanco, Burnet, and other counties of the Llano uplift area. Some galena has been mined at Silver Creek in northwestern Burnet County. Here, galena occurs in cracks and as scattered grains in Cambrian limestones and sandstones.
It is probable that much of the galena in west Texas and in central Texas was formed when hot magma forced out solutions containing lead. These solutions moved up through cracks and other openings in the subsurface rocks and deposited the galena in them.
Small amounts of galena, which likely had a different origin, have been found in Fisher, Foard, Hardeman, and Young counties. A little occurs also in rocks associated with salt in a number of the Gulf Coastal Plain salt domes.
Garnet is not one mineral but is the name given to a group of several minerals that are very much alike. In fact, it often is impossible to tell some of them apart without using special laboratory tests.
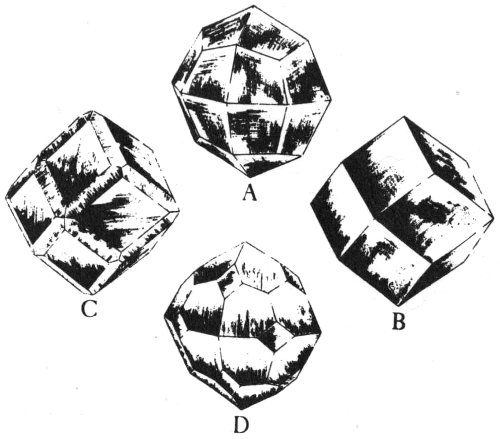
Garnet crystal forms include: A, trapezohedron; B, dodecahedron; C and D, combination trapezohedron and dodecahedron.
The garnet minerals have glassy to resinous lusters and are transparent or translucent. A pocket knife will not scratch them, and some specimens are too hard even for quartz to scratch. Two of the garnet minerals most commonly found in Texas are almandite, an iron-aluminum silicate, and grossularite, a calcium-aluminium silicate. Almandite has a deep-red or a brownish-red color. Grossularite is pale green, brownish yellow, cinnamon brown, or rose red.
Garnet minerals occur as crystals and as masses that are scattered through some of the metamorphic and igneous rocks. After they have weathered out of these rocks, the garnets make up a part of many sands and sandstones. Because these minerals so commonly occur as crystals, it is helpful to learn to recognize the crystal shapes.
Garnet minerals are found in the igneous and metamorphic rocks of both central Texas and west Texas. In central Texas, they occur in ancient Precambrian schist and pegmatite rocks of the Llano uplift area. Some of these central Texas garnet localities are in northeastern Mason County, central and northwestern Llano County, west-central Burnet County, and northeastern Gillespie County.
In west Texas, garnets occur in metamorphic rocks in the Quitman Mountains, which are southwest of Sierra Blanca in Hudspeth County, and in the Mica Mine area, which is south of Van Horn near the Hudspeth-Culberson County line. Garnets also have been found in igneous rocks in the Franklin Mountains a few miles north of El Paso in El Paso County.
Garnets that are found in metamorphic rocks such as schists were formed when great forces squeezed and heated rocks far below the earth’s surface. This heat and pressure caused elements in the rocks to join together into different combinations to form new minerals, such as garnets. Garnets that occur scattered through igneous rocks, such as some pegmatites and granites, cooled and crystallized from hot, igneous fluids when the rocks themselves formed.
Most Texas garnets are not transparent. A few, however, are clear enough to be used as gemstones. These can be cut, polished, 59 and mounted in rings, brooches, bracelets, and earrings. Although some garnet is widely used as an abrasive, none from Texas has been produced for this purpose.
Gneiss is a metamorphic rock that has parallel layers or bands. Some gneiss is made up of the same minerals (chiefly feldspar and quartz) as granite, and it is then called granite gneiss. Several of the other kinds of gneiss are known as mica gneiss, conglomerate gneiss, gabbro gneiss, and hornblende gneiss. In order to be a gneiss, a metamorphic rock has to have bands or layers. These bands may be either straight or wavy and either wide or narrow. In most gneisses, you will find a layer made up of long or flat mineral grains next to a layer made up of the grains of an entirely different mineral. The bands may show color differences, too. For example, a pink layer made up of feldspar grains may be found next to a black layer made up of hornblende grains. The mineral grains interlock as they do in igneous rocks, and they are generally large enough to be seen without a magnifying glass.
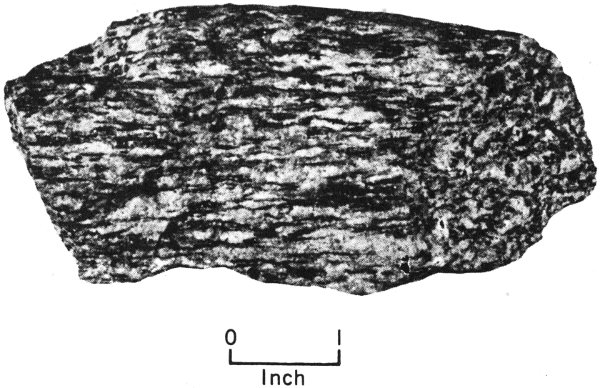
Gneiss from Blanco County, Texas, showing light and dark bands.
Gneiss can form from an igneous rock, such as granite, or from a sedimentary rock, such as sandstone. Heat, fluids, and pressures below the earth’s surface change these rocks into gneiss.
Gneiss that formed during Precambrian time is now seen at the surface in both west Texas and central Texas. In west Texas, it occurs principally in the Van Horn area of Culberson and Hudspeth counties. In central Texas, it is found in Blanco, Burnet, Gillespie, Llano, and Mason counties of the Llano uplift area.
One of the Llano uplift rocks is called the Valley Spring Gneiss. It generally has a light color (much of it is pinkish), and it is believed to have once been a sandstone. Another gneiss of this area, the Big Branch Gneiss, which has a medium to dark gray color, occurs in northern Gillespie and Blanco counties and is an altered igneous rock. Some of the Texas gneiss rocks are suitable for use as building stones.
Gold commonly occurs in nature as a single element—gold—but much native gold has a small amount of some other element, such as silver, copper, or iron, mixed with it.
Native gold is a shiny, yellow, metallic mineral that does not tarnish, and it leaves 60 a shiny, golden-yellow streak when you rub it across a streak plate. If silver is present, the color and streak have a lighter shade. Pure gold is extremely heavy—its specific gravity is 19.3. Because it is malleable, this mineral will flatten into a thin sheet when hammered. It is ductile enough to be drawn out into wires. Gold is also soft—a pocket knife will scratch it easily. When it is to be used for ornaments and jewelry, gold is usually mixed with other metal, such as silver, copper, nickel, or palladium, to make it harder. The amount of gold that is present is then indicated by carats (or karats). Pure gold is 24 carats. If you have a gold ring that has 14 K stamped inside it, you know that it is made of a mixture of 14 parts gold and 10 parts of other metal.
Gold commonly occurs in nature as plates, scales, or grains. Some of the grains are large enough to be called nuggets. It also is found in a wire-like shape described as filiform, it occurs in a network, called reticulate, and it can have a branching and fern-like shape, described as dendritic. Gold is not often found as individual crystals.
Several other minerals, such as pyrite, chalcopyrite, and mica, are sometimes mistaken for gold. None of these, however, is malleable and ductile, and none is nearly as heavy as gold. Pyrite and chalcopyrite have dark-colored streaks unlike that of gold. Mica cleaves so perfectly that it can be split into thin, flat sheets, but gold has no cleavage at all.
The best places to look for gold are in areas near igneous rocks and along the creeks and rivers that drain these areas. It is thought that most gold originally was carried up from molten igneous rock by hot solutions. The solutions moved into cracks and other openings in nearby rocks and deposited the gold, commonly along with quartz. Later, some of these gold-bearing rocks weathered away. The gold that the rocks contained either remained at the spot or was washed into creeks and rivers. These transported accumulations of loose gold are called placer deposits.
No really important gold deposit has ever been found in Texas, although traces and small amounts have been reported in several areas. A little gold has been found in the Llano uplift area of central Texas. It occurs in quartz veinlets that cut through some of the Precambrian metamorphic rocks of Llano, Mason, northeastern Gillespie, and west-central Burnet counties. Many years ago, a small amount of gold was mined northeast of Llano in Llano County from the Heath mine. Some gold also has been found in sands and gravels along streams, such as along Sandy Creek and its tributaries, in parts of this Llano uplift area.
In the Trans-Pecos country of west Texas, small amounts of gold have been found in the Van Horn area of Culberson and Hudspeth counties, in the Quitman Mountains district of Hudspeth County, and in the country around Shafter in Presidio County. Most of the small quantity of gold that was mined in west Texas was obtained as a by-product from the Presidio mine in the Shafter district (described with silver minerals on p. 90).
Small amounts of gold have been reported from other parts of Texas. Some of these localities are in Eocene Tertiary sandstones in the Gulf Coastal Plain, in Cretaceous limestones in Irion, Uvalde, and Williamson counties, and in sand and gravel in Howard and Taylor counties. None of these deposits has been found to have any commercial value.
Granite is an intrusive igneous rock that is made up chiefly of crystalline grains or crystals of quartz and a feldspar mineral, such as orthoclase or microcline. Several other minerals, including mica and hornblende, may also be present.
All of the mineral grains in granite are about the same size, and you can distinguish them without using a magnifying glass. A granite may be coarse grained, medium grained, or fine grained. When you examine this rock, you will see that its grains are not cemented but interlocked like the pieces of a jigsaw puzzle. The color of granite, which is pink, red, gray, or brownish, depends chiefly on the color of its feldspar grains.
Most granites formed from hot, molten magma that slowly cooled and hardened far below the earth’s surface. Because of this slow cooling, fairly large mineral grains were formed.
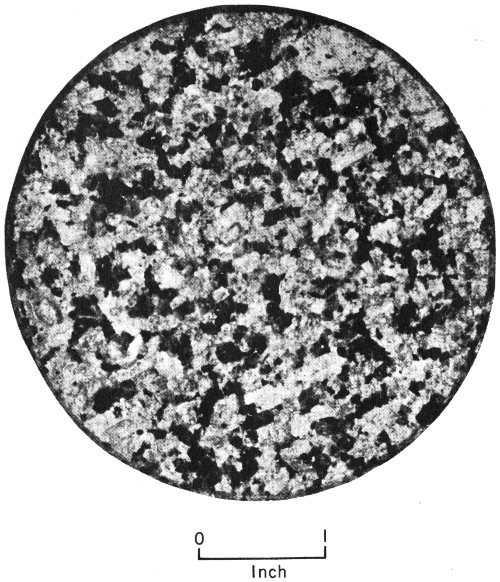
Polished section of pink granite from Gillespie County, Texas.
Granites are now seen at the surface in several areas of Texas. They were gradually uncovered as the areas became higher and the overlying rocks slowly weathered away. One of these areas is the Llano uplift of central Texas where the granites occur in Blanco, Burnet, Gillespie, Llano, and Mason counties. These granites formed during Precambrian time and are believed 62 to be about a billion years old. (Scientists are now able to determine the age of some rocks accurately by very precisely measuring the relative amounts of isotopes produced by decay of radioactive minerals.)

Texas State Capitol building at Austin is made of Burnet County granite obtained from Granite Mountain near Marble Falls, Texas.
Granites also appear at the surface in the Trans-Pecos country of west Texas. Some of these areas include the Franklin Mountains of El Paso County, the Quitman Mountains of Hudspeth County, the Chisos Mountains of Brewster County, and the Chinati Mountains of Presidio County.
Red, pink, and gray granites from quarries in the Llano uplift area are widely used as building stones and monument stones. A large quarry at Granite Mountain just west of Marble Falls in Burnet County has supplied pink granite for buildings in many parts of the United States. The Texas Capitol building and several other State buildings in Austin are made of this granite.
Graphite is a mineral that is made up of a single element—carbon. (Diamond, although 63 it does not look at all like graphite, is a crystalline form of carbon.) Graphite is a steel-gray or black mineral that commonly has a metallic luster. It is not heavy and is extremely soft. Graphite will soil your fingers and leave a black mark on paper. This mineral cleaves perfectly in one direction and splits into thin flakes that feel greasy.
To help distinguish graphite from molybdenite, a mineral it resembles, you can use a shiny, glazed surface, such as is found on a saucer or a plate, to test its streak. When rubbed across this kind of surface, graphite will leave a black streak, but molybdenite will leave a greenish one.
Graphite commonly occurs as scales, as sheet-like layers, or as compact masses. It may be found mixed with clay or other impurities, and it then looks dull and earthy. Crystals of graphite, which are seldom found, are 6-sided and flat.
Graphite occurs in Llano, Burnet, and other counties in the Llano uplift area of central Texas. One of the Nation’s most important graphite mines is located in the Clear Creek area several miles northwest of Burnet in Burnet County. Some graphite has also been mined near Lone Grove in Llano County. In addition, a graphite schist, obtained south of Llano in Llano County, has been used as a filtering material.
Graphite is used in pencil lead, generator brushes, and lubricants.
All of this graphite occurs in extremely old Precambrian graphite schist rocks that we now see at the surface in this part of Texas. It is believed that the schists were once ancient sedimentary rocks, such as shales, which contained organic matter. Long ago, great forces below the earth’s surface altered these rocks. When this happened, the organic material that they contained changed into the mineral we know as graphite.
Graphite has a number of uses. It is mixed with clay to make the pencil lead that we use for writing. It serves as a lubricant, either alone or mixed with oil, grease, or water. In addition, graphite is used to make generator brushes, stove and shoe polish, and special paints. Because it can stand great heat without melting, some graphite is mixed with clay to make the pots or crucibles that hold molten metals.
Grossularite. See Garnet.
Gypsite. See Gypsum.
Gypsum is a hydrous calcium sulfate. This mineral is normally colorless or white, but impurities cause it to appear gray, brownish, yellowish, or reddish. It is transparent or translucent and is not heavy. When you rub gypsum across a streak plate, it leaves a white streak. This mineral is so soft that a fingernail scratches it easily. Gypsum occurs in several varieties.
The colorless, glassy, and transparent variety of gypsum is called selenite. It is found as cleavable masses and as crystals that are prism-shaped or flat and diamond-shaped. It is not uncommon for two crystals to be joined together so that they have a swallow-tail shape—these crystals are twinned. Groups of flat selenite crystals arranged together so that they resemble flowers are called rosettes. Many of these have been found in Nolan County.
Gypsum has four directions of cleavage. One of these directions is so perfect that some selenite splits into thin, clear sheets that may be mistaken for mica; other selenite cleavage fragments may be mistaken 64 for calcite. You can distinguish selenite sheets from calcite by testing their hardness (selenite is softer) and by putting a drop or two of dilute hydrochloric acid on them. The acid will fizz and bubble on calcite but not on the selenite gypsum. There is also a quick way to distinguish the thin selenite cleavage fragments from mica. After you carefully bend a thin sheet of mica, it will snap back to its original shape without breaking. Selenite gypsum, however, is not elastic. It will bend, but it will break if you try to straighten it again.
Selenite is found in cracks and cavities in rocks. Good crystals have been collected at Gyp Hill, a salt dome southeast of Falfurrias in Brooks County, and some selenite has been mined there. Selenite crystals also occur scattered through clays, particularly along creek banks, in Lee, Fayette, Bastrop, and several other counties.
Another variety of gypsum is known as fibrous gypsum. It is made up of slender, brittle, needle-like fibers that fill the cracks in some rocks. If fibrous gypsum has a silky or pearly luster, it is called satin spar. One of the places where satin spar occurs is in Permian rocks in Hardeman County.
Most of the fibrous gypsum and selenite is formed by solutions. Some of these solutions develop when underground waters, seeping through rocks, pick up and dissolve minerals that contain sulfur (such as pyrite). This dissolved material changes the water into very weak sulfuric acid. 65 When the sulfuric acid meets calcium carbonate (as in limestone or calcite), it combines with the calcium to form the gypsum.
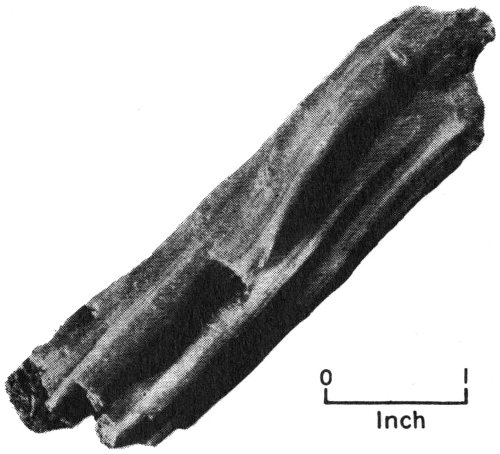
Fibrous gypsum from Terlingua area, Brewster County, Texas.
A massive, fine-grained, and translucent variety of gypsum, known as alabaster, is used for articles such as lamp bases, statuettes, vases, and book-ends.
A loose, earthy, crumbly variety of gypsum, called gypsite, is ordinarily found mixed with other materials, such as clay, sand, and soil. It occurs either at or near the surface of the ground. Gypsite is found in Culberson, Reeves, and other counties in west Texas.
A massive, granular variety of gypsum, called rock gypsum, may occur in large deposits. This is the gypsum that is used for making products such as plaster, wallboard, and some cements.
Deposits of rock gypsum are found both underground and at the surface in Texas. Surface deposits occur in Permian rocks in several counties to the east of the Texas High Plains. They also occur in the area between the Pecos River and the Delaware and Apache Mountains in Culberson and Reeves counties. Some of the other surface deposits are found near the Malone Mountains in Hudspeth County and in Lower Cretaceous rocks in Gillespie and Menard counties. Rock gypsum has been mined from the deposits in Fisher, Gillespie, Hardeman, Hudspeth, and Nolan counties. It also has been produced from the cap-rock at Hockley salt dome in Harris County.
Gypsum and another mineral, anhydrite, have very nearly the same composition. Both are calcium sulfates. Gypsum, however, contains water of crystallization, and anhydrite does not. It is likely that most of the rock-gypsum deposits of Texas originally were beds of anhydrite. By absorbing water that seeped through it, the anhydrite changed into gypsum.
Halite, sodium chloride, is the table salt you sprinkle on food for seasoning. This mineral ordinarily is white or colorless, but other materials cause it to be tinted red, blue, gray, brown, or green. When you rub halite across a streak plate, it leaves a white streak.
Because halite cleaves in three directions, all at right angles to each other, the cleavage fragments are shaped like cubes. You can see some of them by looking at a few grains of table salt through a magnifying glass.
Halite has a salty taste and dissolves easily in water. It also is transparent to translucent and has a glassy luster. This mineral is soft enough for a copper penny to scratch it. Halite commonly occurs as cubic crystals and as granular or compact masses.
In addition to its use as table salt, much halite goes to make soda ash, chlorine, and other chemicals. A few of its other uses are in leather making, meat packing, and food canning.
Texas has large underground deposits of halite. These deposits, known as rock salt, occur in the Permian subsurface basin of west Texas and in the salt domes of the Gulf Coastal Plain. The Permian basin, which extends under parts of west Texas, New Mexico, Oklahoma, Colorado, and Kansas, is now completely filled with sediments. It appears level and flat when you travel across it and does not look at all like a basin or a valley. During Permian time, however, this area was covered by a salty 66 sea. As the sea gradually dried up, the dissolved material that it contained was deposited as thick beds of halite, anhydrite, and other minerals. Later, these minerals were covered by sedimentary rocks which were deposited on top of them. Now, the minerals are found many hundreds of feet below the surface. In Hutchinson, Mitchell, Ward, and Yoakum counties, some of this Permian basin salt has been produced (as brine) from wells that have been drilled into it.
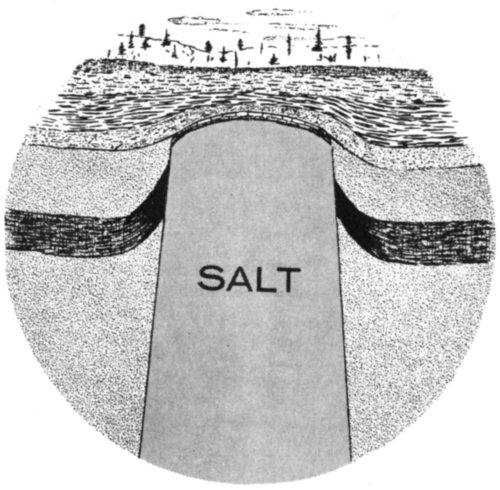
Salt domes, which are huge, underground columns of halite, occur on the Gulf Coastal Plain.
The Gulf Coastal Plain salt domes are huge and almost circular columns of halite, some of which are more than 2 miles wide. Some are less than 300 feet below the surface, but most of them are much deeper. These salt columns pushed upward many thousands of feet from great, deeply buried salt deposits. The halite is mined from shafts dug into the Hockley salt dome in Harris County and into the Grand Saline salt dome in Van Zandt County. Salt brines are produced from wells drilled into several salt domes of this area.
At the surface in Texas, halite occurs in salt lakes in Crane and Hudspeth counties and in alkali lakes on the High Plains. It is found also on the shores of bays and lagoons in Cameron, Kenedy, Kleberg, and Willacy counties, and it occurs at springs and seepages in various places in the State.
Hematite, iron oxide, the chief ore of iron, is found in many places in Texas but not in large deposits. This mineral may 67 have a metallic luster and appear reddish brown, dark brown, steel gray, or black or it may occur as a soft, red, earth-like material called red ocher.
Specular hematite from Carrizo Mountains, Hudspeth County, Texas.
Most metallic hematite is too hard for a pocket knife to scratch, but quartz or a steel file will scratch it. Hematite is fairly heavy, for it has a specific gravity of 5.26. This mineral has no cleavage, but some specimens show three directions of parting that are almost at right angles to each other. A great help in identifying hematite is the dark reddish-brown streak it leaves when you rub it across a streak plate.
Some hematite occurs as rounded masses that resemble kidneys or bunches of grapes (then called kidney ore); it also is found as flat crystals. Most of the Texas hematite occurs as granular or compact masses. One of these massive varieties is composed of shiny scales or plates and is called micaceous or specular hematite. This variety has been found in Hudspeth County and in northeastern Mason County. Hematite also commonly occurs as cementing material in many Texas sandstones.
Some hematite is formed by the alteration of magnetite, another iron mineral. This hematite is known as martite, and some of it still has the crystal shape (an octahedron or a dodecahedron) that belonged to the magnetite. Most of the hematite found in the Llano uplift area of central Texas is believed to be altered magnetite. In this central Texas area, some massive, granular martite has been mined at the Gamble prospect, a few miles southeast of Fredonia in northeastern Mason County, where it occurs as layers in Precambrian gneiss.
Small deposits of hematite occur in other parts of Texas, too. Some of the west Texas localities include Sierra Blanca, the Quitman Mountains, and the Carrizo Mountains of Hudspeth County and the area around Shafter in Presidio County.
Hollandite. See Manganese Minerals.
Hyalite. See Opal.
Jasper. See Quartz.
Kaolin. See Clay.
Limestone is a sedimentary rock made up chiefly of calcite, a calcium-carbonate mineral. This rock also commonly contains grains of quartz, clay minerals, the mineral dolomite, or other materials. If a large amount of dolomite is present, the rock is called dolomitic limestone. In some limestones, the mineral grains are too small to be distinguished from each other without a magnifying glass or a microscope, but in other limestones, the individual mineral grains are easily seen.
Pure limestone is white, but if it contains clay or plant or animal matter it is light gray, dark gray, or black. Limestone also may be some shade of yellow, brown, or red. It is fairly soft and can be scratched with a knife. Because this rock contains calcite, an easy chemical test will help identify it: a drop or two of dilute hydrochloric acid will quickly fizz and bubble when placed on the limestone.
Limestones form in fresh water, such as in lakes, but most of them form in the seas. As some earlier-formed rocks are weathered, the calcium minerals that they contain are dissolved. Creeks and rivers carry this dissolved material to the sea. There, small animals, such as corals, crinoids, sponges, and foraminifers, take the dissolved material out of the water to build their calcium carbonate shells. Plants, such as algae, can take calcium carbonate out of solution too, and it collects on them. Shells, shell fragments, and plant remains accumulate on the sea floor, forming limy deposits that later become limestone.
Limestones also originate in a slightly different way. When the temperature and chemical composition of the water permit, calcium carbonate precipitates as millions of tiny grains of calcite and forms a limy mud that is converted to limestone. Many limestones contain shell or plant fragments in addition to these tiny grains of calcite.
Polished section of Lower Cretaceous Edwards Limestone from Travis County, Texas, containing fossil gastropods.
There are several special kinds of limestone. If the rock is made up of many little rounded calcite grains that resemble fish 69 eggs, it is called öolitic limestone. Another limestone, chalk, is soft, white, and fine grained. It consists mostly of tiny shell fragments and fine-grained calcite. Coquina is a porous limestone made up of loosely cemented shells and shell fragments. Another special kind of limestone, known as lithographic limestone, because it can be used in printing, is smooth, firm, and hard. Its mineral grains are too small to be recognized without a microscope. This kind of limestone breaks with a smooth, sometimes curved, fracture. Still another variety, pulverulent limestone, is loose, soft, powdery, and white. It occurs in the Lower Cretaceous Edwards Limestone in Williamson and Bell counties of central Texas. Some of this limestone is used to polish rice grains, and it is added to livestock feeds to provide calcium for the animals.

Limestone quarry in Lower Cretaceous Edwards Limestone at Georgetown, Williamson County, Texas.
Much limestone is found at the surface in Texas in Cambrian, Ordovician, Mississippian, Pennsylvanian, Permian, and Cretaceous formations. If you will look at numbers 5, 6, 9, 10, and 11 on the Texas geologic map (pp. 4-5), you will see that these strata appear at the surface in central, north-central, and Trans-Pecos Texas.
Limestone has many important uses. Much Texas limestone is crushed and used as a road-building material and as an aggregate that is mixed with cement to make concrete. Farmers in some areas improve their crops by adding limestone to the soil. Limestone also is sent to the iron furnaces in east Texas to be used in the production of pig iron and steel.
Some of the Texas limestones are heated to a fairly high temperature in order to change them into lime (calcium oxide). Industry uses a large amount of lime in making chemicals, steel, glass, paper, and other products. Builders use it to make plasters, mortars, and stuccos. At plants in Comal, Johnson, Travis, and Williamson counties, lime is made from Cretaceous limestones.
Another important use of limestone is in making portland cement. The limestone is 70 mixed with clay or shale, and the mixture is burned in a kiln until it just begins to melt. Then it is allowed to cool. Next, it is finely ground and in order to keep the finished cement from hardening or setting too quickly when it is used, a retarder, such as gypsum, is added. A number of cement-manufacturing plants in Texas use Cretaceous limestones, shales, and clays.
Many of the Texas limestones make excellent building stones. Some of them are quarried from Pennsylvanian and Cretaceous formations in north-central Texas and from Lower Cretaceous formations in counties near the Llano uplift of central Texas. A large quarry on the Williamson-Travis County line near Cedar Park in central Texas has supplied Cretaceous limestone for many buildings and monuments in the United States and Canada.
Limonite is not really a definite mineral but is a mixture of iron oxides containing water. It is believed to be closely related to an iron mineral called goethite. Some limonite may be dull and earthy with the appearance of brownish-yellow or rusty brown clay. This variety is so soft that a fingernail will scratch it easily.
Other limonite has a dark brown or black color and a metallic or almost metallic luster. A copper penny will not scratch it, but a steel file will. This kind of limonite may have a shiny black surface that resembles glossy lacquer. The property that will help you most in identifying limonite is the rusty, yellowish-brown streak it leaves when rubbed across a streak plate.
Limonite has no cleavage and no crystal shape of its own. But crystals of other iron minerals, such as pyrite and magnetite, alter to form limonite. It then occurs with a crystal shape that originally belonged to one of these other minerals. (Such false forms of minerals are called pseudomorphs.) Limonite also occurs as layers in rocks, as hollow or solid concretions, or as coatings on other minerals. It is found mixed with minerals such as clays and serves as the cementing material in some sandstones.
Limonite is found in many localities in Texas including Blanco, Brewster, Burnet, Llano, and San Saba counties. The most important limonite deposits in Texas, however, are in the eastern part of the State, particularly in Anderson, Cass, Cherokee, Henderson, Marion, Morris, Nacogdoches, Smith, and Upshur counties.
The east Texas limonite deposits occur mainly in Weches sedimentary rocks. These rocks, which were deposited in the sea during Eocene Tertiary time, contain clay along with greensands. (Greensands are small, soft grains that contain glauconite, a mineral composed of iron, silicon, and several other elements.) Later, as the sea retreated, these sediments became a part of the land. Waters seeping through the sediments changed into weak solutions of carbonic and sulfuric acid that dissolved the iron out of some of the greensands. When conditions were favorable, this iron was re-deposited as an iron-carbonate mineral called siderite. Siderite was changed to limonite by weathering. Some siderite is still found in east Texas, and it is also mined along with the limonite as an iron ore.
East Texas iron ore has been mined from time to time ever since about 1855, and records show that a number of local iron furnaces once operated. The brown iron ore (as the limonite is also called) now is mined from open pits in Cass, Cherokee, and Morris counties.
This ore, after being washed, goes into blast furnaces at Lone Star (near Daingerfield) and at Houston. In the blast furnaces the ore is changed into metallic iron by mixing it with coke (made from coal) and limestone and blowing in blasts of hot air.
To make steel, the iron from the blast furnace (called pig iron) is put into open-hearth furnaces together with scrap iron, limestone, and other materials. This mixture is heated and melted together to get rid of unwanted substances. Then other elements, such as molybdenum, manganese, 71 or nickel, are added to make steel with the right strength and toughness.
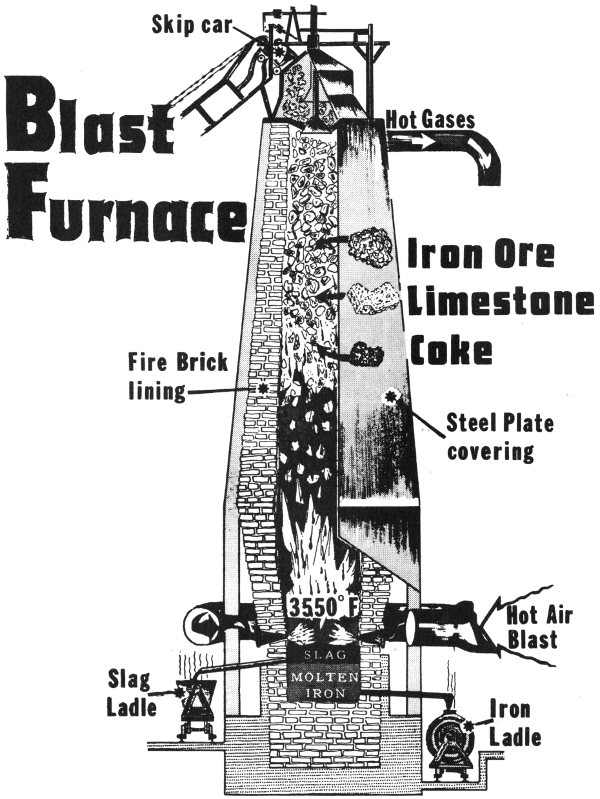
Limonite ore is changed to metallic iron in a blast furnace.
Steel mills alongside the furnaces in Texas turn out many products, such as steel plates for oil tanks, ships, and tank cars and steel beams for framework in buildings and bridges. Some of their other products include pipes for the oil and chemical industries and wire for nails and fencing material.
Lithographic Limestone. See Limestone.
Llanite is a unique rock that is found only in Llano County in central Texas. 72 This intrusive igneous rock is made up of easily seen crystals and grains of quartz and feldspar that are scattered through a brown-colored mass of extremely small mineral grains. The quartz is beautiful, sky-blue, and opal-like; the feldspar has a rusty pink color. (Because the quartz looks like opal, this rock often is called opaline granite.) The mineral grains that make up the brown-colored mass are so tiny that they can be identified only with a microscope. They are quartz, feldspar, mica, fluorite, and apatite.
Llanite formed during Precambrian time. Molten rock material forced its way upward into cracks that cut across granite and schist rocks while the rocks were still far underground. This hot magma remained in the cracks where it cooled and hardened to form long, narrow, wall-like masses (called dikes) of llanite. We can see some of the llanite dikes exposed at the earth’s surface to the north and northeast of Llano in Llano County because the overlying rocks have weathered away.
Llanite has been quarried from one of the dikes west of Babyhead in northern Llano County. Because llanite is both attractive and strong, it has been used as an ornamental stone and as a monument stone.
Magnetite, iron oxide, is a black, metallic mineral with an outstanding physical property: it is magnetic—fragments of magnetite readily cling to a magnet. It also leaves a black streak when rubbed across a streak plate. Although this mineral is too hard to be scratched by the average pocket knife, a steel file will scratch it. Magnetite is fairly heavy—it has a specific gravity of 5.18.
Magnetite occurs as compact or granular masses, as scattered grains, and as crystals. Most of the crystals are octahedrons, but some dodecahedrons are found. Magnetite helps make up a part of many metamorphic and igneous rocks, and it also occurs as tiny crystals and grains in some sands, sandstones, and other sedimentary rocks.

Metallic iron, after leaving the blast furnace, is made into steel in an open-hearth furnace.
Most of the magnetite that has been found in Texas occurs in Precambrian gneiss and schist rocks of the Llano uplift area of central Texas, particularly in Llano County and in eastern Mason County. It occurs as thin layers, as thick lens-shaped deposits, and as scattered grains in the rocks. Probably at least a billion years ago these gneisses and schists were sedimentary rocks, such as shales and 73 sandstones. Some geologists believe that these rocks could have contained iron sediments (perhaps in the form of glauconite). Great forces below the earth’s surface crumpled and squeezed the sedimentary rocks and changed them into the metamorphic schist and gneiss rocks we see today. As this happened, the iron sediments in the rocks were changed into magnetite.
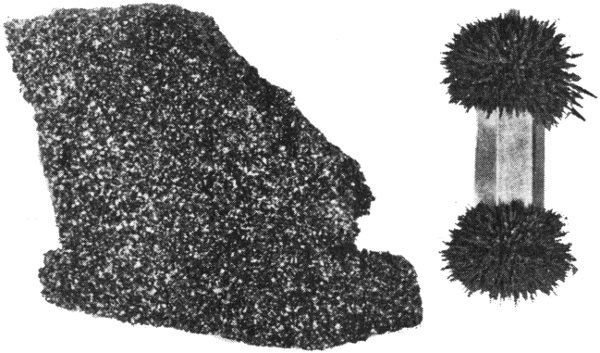
Granular magnetite fragments from northwest of Llano, Llano County, Texas, are attracted to a magnet.
At least some of the magnetite in this area (such as the deposit at Iron Mountain in Llano County) probably had a different sort of origin. Molten igneous rock material containing iron could have moved up into cracks in the ancient sedimentary rocks. Then the magnetite formed from this iron material when the igneous and sedimentary rocks were changed into the schists and gneisses of today.
None of the Llano and Mason County magnetite deposits is really very large. Nevertheless, prospecting and a little mining have been carried on from time to time at several deposits in this area. At Iron Mountain, which is about 12 miles northwest of Llano in Llano County, magnetite has been mined from open pits. Although magnetite is commonly used as a source of iron, the magnetite from this deposit was used as a heavy concrete aggregate.
Malachite. See Copper Minerals.
Although manganese does not occur alone in nature as a native element, it makes up a part of many minerals and compounds. This element has an important use in steel making, where it helps rid the steel of unwanted substances, such as oxygen and sulfur, and, in addition, it is used to make tough, hard, manganese steel for armor plate, railroad tracks, safes, and steam shovels. Manganese has various uses outside the steel industry. It is added to copper and nickel to make alloys, it is used in the manufacture of dry-cell batteries, and (as manganese sulfate) it is used as a fertilizer.
Manganese minerals and compounds, such as braunite, hollandite, pyrolusite, and wad, occur in several counties in Texas. No large, commercial deposits have been found here.
Some manganese compounds and minerals are covered with a soft, sooty black material that will soil your fingers. This can help you recognize these minerals; however, a few non-manganese minerals, such as some chalcocite, also have a black coating that soils your fingers in a similar way.
One of the manganese minerals, braunite, is a complex oxide of manganese that contains silica. It has a submetallic luster and is dark steel-gray or black. When rubbed across a streak plate, it leaves a steel-gray or a black streak. This mineral is too hard to be scratched by a pocket knife, but a piece of quartz or a steel file will scratch it. Braunite has a specific gravity of 4.75 to 74 4.82. It has four directions of cleavage that are parallel to the faces of a pyramid.
In the Spiller mine, about 15 miles northeast of Mason in Mason County, masses of braunite occur as lens-shaped layers in Precambrian gneiss and quartzite rocks. This braunite may have formed from another manganese mineral (possibly manganese garnet) that was exposed at the earth’s surface after the overlying rocks eroded away. As this other mineral weathered, it may have altered into braunite, or the braunite could have been deposited from solutions emanating from hot magmas before the great thickness of overlying rock was removed.
The mineral variety hollandite is a rare manganate of manganese and barium. It has a metallic luster, and its color is silvery gray or black. When you rub it across a streak plate, hollandite leaves a black streak. It has a specific gravity of 4.7 to 5. Hollandite is rather hard, but a steel file will scratch it.
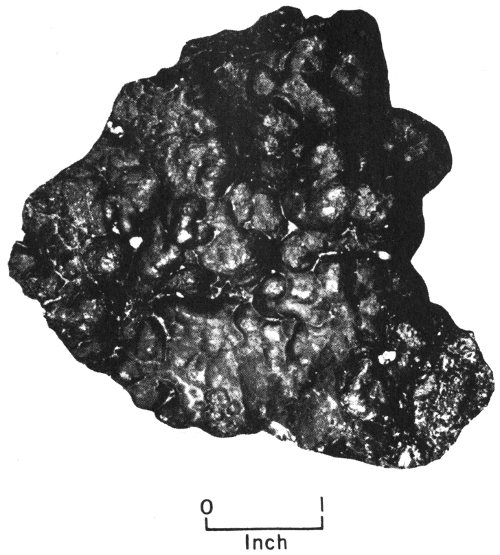
Hollandite from Jeff Davis County, Texas.
Hollandite occurs in western Jeff Davis County in west Texas at what is called the Mayfield prospect. Here, it is found as rounded masses that occur in a vein near a large fault in Lower Cretaceous limestone rocks.
Other manganese compounds, pyrolusite and wad, are found in several important deposits near the Pecos River in western Val Verde County. Pyrolusite is a manganese dioxide mineral. It is black, opaque, and so soft that it rubs off on your fingers like soot. Pyrolusite may be granular and massive or may be powdery. It also occurs as a fern-like coating on rocks. Wad is not really a mineral but is an impure, dull-black or brownish-black mixture of manganese oxide, water, and other substances. It can be soft enough to soil your fingers, or it can be too hard to scratch with a 75 pocket knife. Wad occurs in earthy or compact masses or in crusts or stains on rocks.
In Val Verde County, the wad and pyrolusite are found mixed with soil, clay, gravel, sand, and plant remains. This material fills cracks in Lower Cretaceous limestones, it is scattered through gravels, and it is deposited in low places at the surface. The manganese in these deposits came from limestone rocks that have since weathered away. Rainwater trickled into these rocks and dissolved the manganese minerals they contained. This manganese was washed down toward the Pecos River and was deposited as wad and pyrolusite.
Marble is a metamorphic rock made up chiefly of sparkling grains of calcite or dolomite, but other minerals may be present. The marble may be fine grained, medium grained, or coarse grained; commonly, all the mineral grains are about the same size.
Marble may be of uniform color, banded, spotted, or streaked. If it is made up only of pure calcite or dolomite, the marble is white. If, however, it contains carbonaceous material, such as graphite, it is grayish or black. Limonite impurities cause the marble to be yellowish brown, and manganese oxides and hematite give it a brownish, pinkish, or reddish color.
Marble is a rather soft rock, and you can scratch it easily with a pocket knife. A few drops of dilute hydrochloric acid will bubble and fizz readily on calcite marble; on dolomite marble, it may fizz slightly.
Marble forms from limestone or from dolomite rock. Heat and pressure below the earth’s surface cause the calcite and dolomite mineral grains in these rocks to recrystallize. A fine-grained limestone can be changed into a coarse-grained calcite marble. The marble is not made up of new and different minerals, but it has a new texture unlike that of the limestone. (To a builder, the word marble has another meaning. He considers rocks such as unaltered limestone, unaltered dolomite, or even serpentine to be marble, if they will take a high polish.)
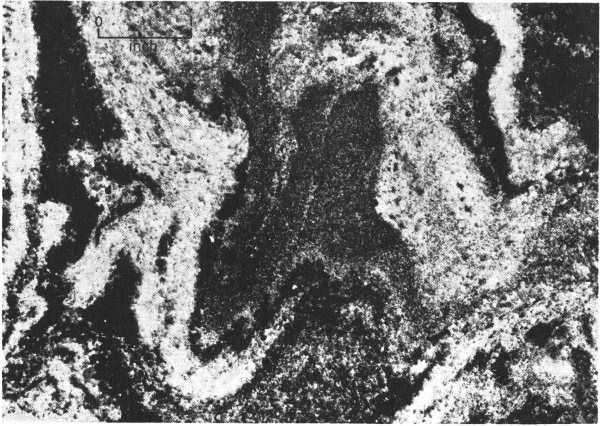
Polished section of Precambrian metamorphic marble from Llano County, Texas.
Metamorphic marbles occur at the surface in central Texas and in west Texas. Some of the west Texas occurrences are in the Van Horn area of Culberson and Hudspeth counties and in the Big Bend 76 area of Brewster County. In central Texas, Precambrian marbles are found in Burnet, Gillespie, Llano, and Mason counties of the Llano uplift area. Many of them are suitable for use as monument and building stones. Some of the Llano County marble is quarried and used as granules for roofs and as terrazzo chips for making colorful floors (described with serpentine on p. 88).
Martite. See Hematite.
Mica is not just one mineral but is the name given to a group of similar minerals. The mica minerals are easy to recognize. Because they have perfect cleavage in one direction, they split into thin, flat sheets. You can see through some mica sheets, and they are elastic enough to be bent back and forth. (Another mineral, selenite gypsum, also will split into thin, flat, transparent sheets, but selenite sheets break when you bend them.)
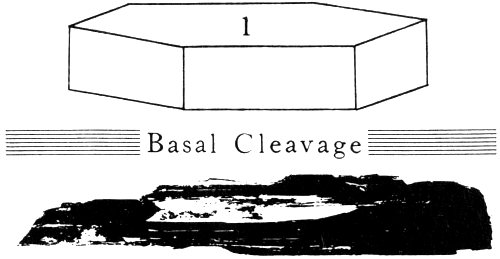
Mica minerals have perfect cleavage in one direction, resulting in thin, sheet-like cleavage fragments.
Two of the mica minerals that you are most likely to find in Texas are muscovite and biotite. Both these minerals are potassium-aluminum silicates, and biotite, in addition, contains magnesium and iron. In general, muscovite is light colored, that is, it has a light brown, yellow, or green tint, or is colorless, and biotite is dark colored, commonly dark green, brown, or black. These minerals have glassy or pearly lusters and are rather soft—a copper penny scratches them. The specific gravity of biotite is 2.8 to 3.2, and that of muscovite is 2.76 to 3.1.
Mica minerals occur in igneous rocks, such as granite and pegmatite, and in metamorphic rocks, such as schist and gneiss. They also are found as tiny flakes in some sandstones, limestones, and other sedimentary rocks. Most of the Texas mica is found in the Llano uplift area (particularly in Llano County) and in the Mica Mine area. (The Mica Mine area is in the Van Horn Mountains about 15 miles south of Van Horn in west Texas.) In both these areas, the mica minerals occur mostly in Precambrian pegmatites and mica schists.
The gleaming mica schists were once igneous rocks or sedimentary rocks such as sandstones and shales. Long ago, great forces beneath the earth’s surface changed the rocks into mica schists. The mica that is found in pegmatites formed from hot fluids of igneous origin when the pegmatite rock itself was formed.
Clusters of mica in the pegmatites are called books, because the thin sheets into which the mica splits look like pages. Some muscovite books up to 8 inches across are found in the Mica Mine area of the Van Horn Mountains.
The books or sheets of muscovite mica that occur in pegmatites are especially valuable to industry. Muscovite can stand great heat without melting, it is tough, it splits into thin sheets, and it lets very little heat and electricity pass through. Because of these properties, muscovite is used in fuses and as insulators in heating elements of electric irons and toasters. (Biotite is not used, because the iron it contains makes it a conductor of electricity.) Sheet muscovite also is widely used by the electronics industry as a non-conducting material in the manufacture of tubes and other products.
Both muscovite and biotite from mica schist rocks, as well as scrap pieces of sheet mica from pegmatites, are ground into flakes or powder. This ground-up mica has many uses, ranging from a powder coating 77 for automobile inner-tubes to Christmas tree “snow.”
Only a small amount of mica has been mined in Texas. A fair grade of sheet mica occurs in the pegmatites at Mica Mine in west Texas, but the deposit is not large. In the pegmatites of the Llano uplift area of central Texas, no sheet mica has been found that is considered good enough for the requirements of industry. Mica suitable for grinding, however, is found in both these Texas areas.
Micaceous Hematite. See Hematite.
Microcline. See Feldspar.
Milky Quartz. See Quartz.
Muscovite. See Mica.
Native Silver. See Silver Minerals.
Obsidian is a dark, glassy-looking igneous rock. Most obsidian contains the same chemical elements as granite and rhyolite, since all three of these rocks can form from the same type of molten rock material. Obsidian, however, has no separate minerals, because its chemical elements are not combined in an orderly way. It is a natural glass.
Because it is a glass, we know that obsidian forms very quickly. One way for it to form is from the sudden cooling of hot, molten lava that flows out of volcanoes. If the lava cools and hardens before the separate minerals can crystallize, it becomes a natural glass, such as obsidian.
This rock is smooth and shiny. Most of it is black, but some can be dark green or dark brown. Obsidian allows light to pass through it, and it breaks with a curved, conchoidal fracture. The broken edges are very sharp.
Another glassy igneous rock that forms from fast-cooling lava is vitrophyre. It looks like obsidian except that it has crystals or crystalline mineral grains (which may be light colored) scattered through the dark glassy material.
Obsidian was used by the Indians to make arrowheads.
Obsidian and vitrophyre are found in the Big Bend area of Brewster and Presidio counties in west Texas. They occur with other igneous rocks that formed there during Tertiary time.
The Indians who long ago roamed this area used the smooth, shiny vitrophyre and obsidian to make some of their arrowheads and scrapers. Today, rock collectors pick up these attractive rocks for their collections, and some of them cut and polish obsidian and vitrophyre for use as gemstones.
Onyx. See Quartz.
Öolitic Limestone. See Limestone.
Opal is like hardened jelly or gelatin. It has no crystalline inner structure and no crystal shape of its own—it is amorphous. This mineral has almost the same chemical composition as quartz. Both are silicon dioxides (silica), but opal, in addition, contains water.
Opal can be almost any color—red, yellow, blue, brown, gray, white—or it can be colorless. It is transparent or translucent and appears glassy, resinous, greasy, or dull. Opal has a specific gravity of 1.9 to 2.2—this mineral is a little lighter than quartz. It also is softer than quartz. A copper penny will not scratch opal, but quartz will. Opal has a white streak and a curved, conchoidal fracture but no cleavage.
Opal occurs in a number of places in Texas. In the Trans-Pecos country of west Texas, it fills cracks and cavities in some of the extrusive igneous rocks. It occurs on the High Plains of northwest Texas, and it is found in Tertiary formations of the Gulf Coastal Plain where it occurs as masses that fill cracks and cavities in sedimentary rocks, as the cementing material in some sandstones (such as in the Catahoula sandstone), and as opalized wood.
Much opal forms from underground waters that contain silicon. These solutions move through the rocks and deposit the opal in them.
Opal is found in a number of varieties. Some show a beautiful, lustrous play of colors that comes from inside the specimens. These varieties are known as precious opal and are prized as gemstones. In Texas, some precious opal is found near Alpine in Brewster County. It has a milky white to bluish-white color, is translucent, and shows a fiery orange, red, blue, and green play of colors.
The variety known as common opal shows no play of colors. It may be white, gray, bluish, reddish, greenish, or yellowish, and it is only slightly translucent. It is found in Brewster, Jeff Davis, Presidio, and other counties of the Trans-Pecos country of west Texas. It occurs also around some of the wet-weather (playa) lakes on the Texas High Plains. In the Gulf Coastal Plain, common opal is found with chalcedony (a variety of quartz) in Tertiary formations. A south Texas locality sometimes visited by collectors is near Freer in Duval County.
A clear, commonly rounded, variety of opal that looks like ice is called hyalite. Two areas in which it has been found are in Presidio County in west Texas and in Llano County in central Texas.
Opalized wood from Washington County, Texas.
A variety of petrified wood, called opalized wood, is opal that replaced the fibers of a piece of wood. Wood opal is found at a number of places in the Gulf Coastal Plain. It occurs there in Tertiary formations within about 20 miles of the boundary line between areas 2 and 3 shown on the geologic map (pp. 4-5).
A soft opaline material called diatomite, or diatomaceous earth, is made up chiefly of the skeletons of diatoms—tiny, one-celled plants that live in fresh or salt water. These little plants are able to take silica from the water to make opal skeletons for themselves. When the diatom skeletons collect at the bottom of a lake or sea, they form the light, crumbly, white, gray, or cream-colored deposit of impure opal known as diatomite. Industry uses this material as a filter, as insulation, as an abrasive, and as a filler.
Diatomite formed in ancient lakes on 79 the Texas High Plains during late Tertiary (Pliocene) and early Quaternary (Pleistocene) times. It is found in Armstrong, Crosby, Dickens, Ector, Hartley, and Lamb counties.
Opaline Granite. See Llanite.
Orthoclase. See Feldspar.
Pegmatites occur in igneous rock areas, and most geologists consider them intrusive igneous rocks. They are made up of crystals and crystalline mineral grains that fit together—the grains are interlocked. The crystals and grains in pegmatites are larger than those of surrounding rocks, and some are huge, even larger than a man. However, there is a wide range of grain sizes in pegmatite.
Some pegmatites cut through igneous or metamorphic rocks in such a way that they resemble walls (called dikes). Others are found as veins, as flat masses, or as odd-shaped bodies in rocks. Many pegmatites occur in granites and contain feldspar, quartz, mica, and other minerals, as granite does. Some pegmatites occur with other kinds of igneous rocks and contain the same minerals as these rocks. A few pegmatites contain rare and unusual minerals.
Many geologists believe that pegmatites form from hot fluids of igneous origin that are left after other igneous rocks, such as granite, have already formed. These left-over fluids contain large amounts of aluminum, potassium, silicon, sodium, and several other elements. While the granite or other rocks are still far underground, this material pushes up into them, and may even partly dissolve them. Then it slowly cools and hardens into pegmatite. It is believed that, later, more fluids move into cracks in some pegmatites. This new material adds other minerals to the pegmatites and alters some of those minerals already there.
Quartz-feldspar pegmatite from Burnet County, Texas.
Some of the pegmatites we now see at the surface in Texas are probably about a billion years old. They formed during Precambrian time and occur with other extremely old rocks. One well-known Texas pegmatite area is the Mica Mine district of west Texas. It is about 15 miles south of Van Horn in the Van Horn Mountains of Culberson and Hudspeth counties. Another pegmatite area is in the Llano uplift of central Texas. These central Texas pegmatites 80 occur in Burnet, Gillespie, Llano, and Mason counties.
Large crystals and grains of feldspar, mica, and quartz are found in the pegmatites of both these areas. A small amount of mica has been mined from the west Texas pegmatites, and feldspar has been produced from the central Texas pegmatites.
An extremely rare and unusual pegmatite occurs in the Llano uplift area at Baringer Hill, which is west of Burnet in Llano County. This pegmatite was once on the bank of the Colorado River, but when Buchanan Dam was built, the area was flooded. The Baringer Hill pegmatite now lies beneath the water of Lake Buchanan. Many rare minerals, which contain beryllium, cerium, thorium, uranium, yttrium, zirconium, and a number of other elements, occur in this pegmatite. Some of these minerals, such as those containing yttrium and zirconium, glow or incandesce when they are heated. During the early part of this century, before the area was flooded, several of the yttrium minerals were mined and used in making lamp mantles.
Pitchblende. See Uranium Minerals.
Precious Opal. See Opal.
Pulverulent Limestone. See Limestone.
Pumicite. See Volcanic Ash.
Pyrite is a shiny, pale golden-yellow or brassy-yellow metallic mineral. This mineral, an iron disulfide, is so often mistaken for gold that it is widely known by the nickname fool’s gold.
Except for their similar color and luster, pyrite and gold are really very different. When you rub pyrite across a streak plate, it leaves a black, a greenish-black, or a brownish-black streak, but the streak of gold is gold-colored. Pyrite is too hard for the average pocket knife to scratch, but a knife will scratch gold easily. Pyrite is brittle and readily breaks, but gold is malleable and flattens out when hit with a hammer. Pyrite is only about 5 times as heavy as an equal volume of water, but pure gold is over 19 times as heavy. And pyrite may have a brown or a multicolored tarnish on it, but gold never tarnishes.

Cubic crystals of pyrite.
Pyrite is a common mineral and is found in many of the igneous, metamorphic, and sedimentary rocks of Texas. It may be scattered through the rocks, or it may fill cracks and cavities in them. This mineral occurs as granular and compact masses, as rounded masses, or as crystals. The crystals are commonly cubes, pyritohedrons, or 81 octahedrons. In some crystals, the shapes are combined (such as a cube with an octahedron or two pyritohedrons grown through each other). You may notice that the sides of some cubes and pyritohedrons have fine, parallel grooves (called striae or striations) on them.
Pyrite originates in a number of different ways. Some of it forms, along with other minerals in igneous rocks, from hot magmas. It also forms in metamorphic rocks by the same processes that produce these rocks. Some of the pyrite in limestone and other sedimentary rocks is formed when the rocks themselves are deposited by seas or streams. Pyrite also is deposited by the hot fluids that are given off by magmas. These fluids travel up into cracks and other openings in rocks and then form pyrite as well as other minerals. Much pyrite forms in still another way. As water seeps through rocks, it dissolves some of the iron minerals that they contain. When, under certain conditions, these iron solutions mix with hydrogen sulfide (this is the gas that makes some water smell like rotten eggs), pyrite is formed.
Pyrite alters easily. Because of this, most builders carefully check the limestone, granite, marble, or whatever other building stone they plan to use to be sure that it does not contain large amounts of pyrite. When exposed to the weather, pyrite changes to limonite and causes an unsightly rust stain.
Pyrite is used as a source of sulfur, and it is produced for this purpose in several states. In Texas, however, no pyrite deposits have been found that are large enough to be mined.
Pyrolusite. See Manganese Minerals.
Quartz, silicon dioxide, is one of the most common minerals. It is glassy, waxy, greasy, or dull and is transparent or translucent. Pure quartz is colorless, but impurities make some varieties white, black, or a shade of red, yellow, blue, violet, or brown. Quartz is a hard mineral. It scratches window glass and cannot be scratched by a pocket knife or even by a steel file. It has a specific gravity of 2.65. The curved, conchoidal fracture shown by many specimens helps identify it.
Quartz is plentiful in Texas. It occurs in igneous rocks, such as granite, llanite, and pegmatite; in metamorphic rocks, such as quartzite, schist, and gneiss; and in sedimentary rocks, such as some sandstone, conglomerate, and breccia.
Quartz crystal, with inclusions, from Burnet County, Texas.
Quartz is found as crystals and as masses. Some of the masses are coarsely crystalline, but some are made up of extremely small crystalline particles called cryptocrystalline quartz. Some of the cryptocrystalline varieties of quartz found in Texas are chalcedony, chert, and jasper. Some of the coarsely crystalline varieties 82 found here are amethyst, milky quartz, rose quartz, smoky quartz, and rock crystal.
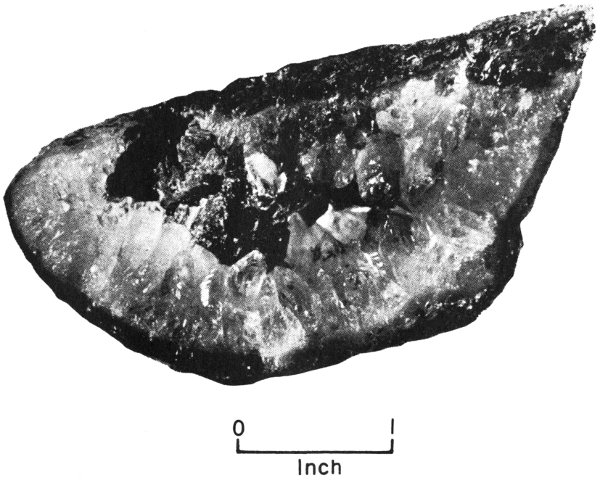
Amethyst geode from the Alpine area of Brewster County, Texas.
A colorless, glassy variety of quartz, called rock crystal, is clear enough to see through. It is found as crystals that are 6-sided prisms with pyramid-like faces on the ends. This variety is commonly associated with igneous rocks, such as those of the Llano uplift area of central Texas and of the Trans-Pecos country of west Texas. It is commonly used as a gemstone and is made into necklaces, earrings, and other jewelry. Some specimens of rock crystal have slender, needle-like crystals of other minerals, such as tourmaline, actinolite, or rutile, enclosed in them.
A clear, glassy variety of quartz, amethyst, has a purple or violet color. It, like rock crystal, is commonly found in 6-sided prisms with pyramid-shaped ends and is also prized as a gemstone. Amethyst has been found in Precambrian rocks in the Llano uplift area of central Texas. (Amethyst Hill, a locality well known to collectors for many years, is in northeastern Gillespie County.) In west Texas, amethyst has been found in Cenozoic igneous rocks in the Sierra Blanca and Quitman Mountains of Hudspeth County and in the Alpine area of Brewster County.
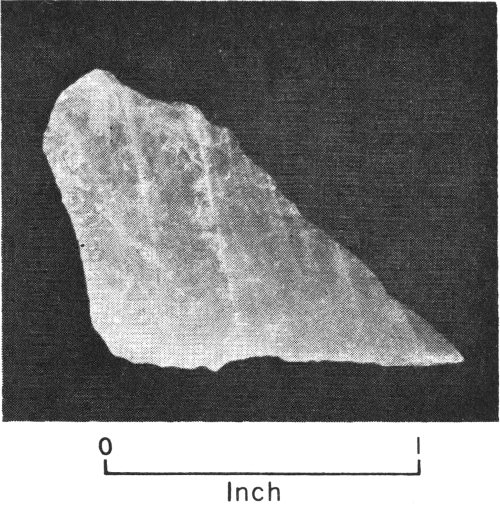
Milky quartz from Burnet County, Texas.
A variety of quartz with a milk-white color and a glassy to greasy luster is called milky quartz. It occurs either as crystals or as crystalline masses. Very little light will pass through it. In central Texas, milky quartz occurs abundantly in the Precambrian 83 rocks of the Llano uplift area in Blanco, Burnet, Gillespie, Llano, and Mason counties. It also is found in some of the rocks of the Trans-Pecos country of west Texas, such as in the Carrizo Mountains of Culberson and Hudspeth counties. Other good places to look for this variety of quartz are in the sands and gravels along many streams in Texas.
Some quartz has a glassy to a greasy luster and a rose or pink color. Rose quartz, as this variety is called, commonly occurs as masses rather than as individual crystals. It can be found along some of the streams in Texas and also in igneous rocks, such as those of the Llano uplift area of central Texas.
A kind of quartz with a smoky brown, a smoky yellow, or a dark brownish-black color is called smoky quartz. Its luster is glassy, and it may be either translucent or transparent. Smoky quartz is commonly found as crystals that are shaped like 6-sided prisms with pyramid-like ends. It is commonly associated with igneous rocks, and beautiful specimens have been found in the Lake Buchanan area of Llano and Burnet counties in central Texas.
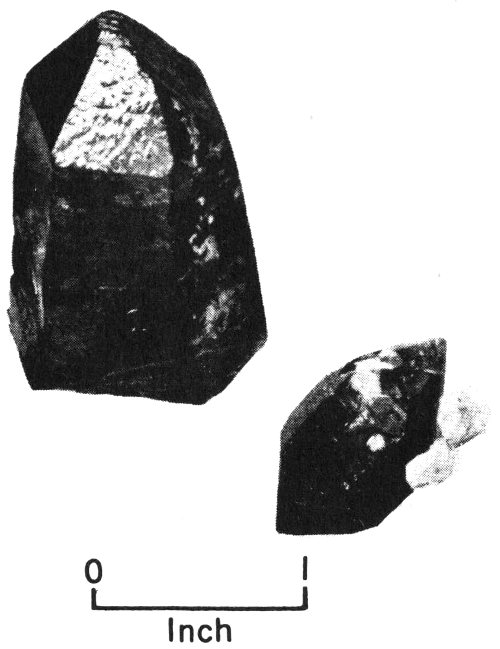
Smoky-quartz crystals from Burnet County, Texas.
A cryptocrystalline variety of quartz, chalcedony, has a waxy to dull luster and a tan, white, gray, or light-blue color. It is translucent but not transparent. Chalcedony does not have its own crystal shape but instead is found in masses that line or fill cracks, pores, and other cavities in rocks. It is formed when water containing silicon slowly seeps into these openings in the rocks and deposits the silicon dioxide there as chalcedony.
Chalcedony commonly occurs in some of the Tertiary rocks of the Gulf Coastal Plain. For example, chalcedony associated with opal is found near Freer in northern Duval County. In the High Plains of west Texas, it is found in alkali-lake deposits, such as at Shafter Lake in Andrews County and at Cedar Lake in Gaines County. In the Trans-Pecos country of west Texas, it can be found filling small cavities in extrusive igneous rocks.
A variety of chalcedony that generally is made up of more than one color is called agate (although agates consisting of several 84 shades of a single color are also found). The colors may be spread out unevenly so that the agate has a cloudy appearance, or they can be arranged in wavy, in straight, or in concentric lines or bands. If the bands are straight and parallel, the specimen is called onyx. Agate that has a moss-like or tree-like design in it is called moss agate. Some agates make attractive gemstones when cut and polished.
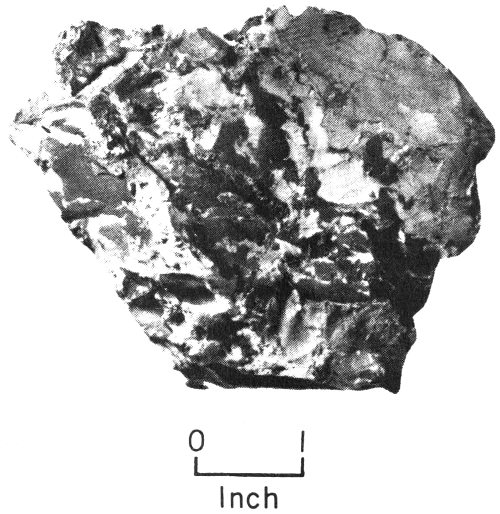
Jasper from Uvalde County, Texas. Dark areas are brownish red; light areas are a yellowish-tan.
Much agate has been found filling cavities in Cenozoic igneous rocks in Brewster, Presidio, and other counties in the Trans-Pecos country of west Texas. It has been found also in an area about 10 to 15 miles wide along the Rio Grande, mostly in southern Webb County and in Zapata and Starr counties.
Trees and other plants have been replaced by agate. Many specimens of agatized wood have been collected from Tertiary formations in Fayette, Gonzales, Lee, Washington, and other counties of the Gulf Coastal Plain. (The agatized wood, along with opalized wood, occurs within about 20 miles of the boundary between no. 2 and no. 3 on the geologic map, pp. 4-5.)
A hard, compact, slightly translucent variety of cryptocrystalline quartz is called jasper. It commonly has a red, brown, or yellow color due to the presence of an iron oxide, such as hematite. Some jasper is made up of irregular bands of more than one of these colors. This variety of quartz often is polished to make attractive gem or ornamental stones. It has been collected at several localities in Texas, particularly from creek and river gravels. Starr and other nearby counties along the Rio Grande have furnished a number of good specimens.
A hard, smooth, compact, translucent rock that is made up mostly of cryptocrystalline quartz is called chert or flint. It is white, black, or some shade of gray, brown, or pink, and its luster is waxy, slightly glassy, or dull. Chert is found in many creek and river gravels in Texas. It also occurs with limestone, such as in the Lower Cretaceous Edwards Limestone of central Texas and in the Ordovician Ellenburger strata in the Llano uplift area. Chert also is found with the Ordovician rocks of the Marathon area of Brewster County.
Geologists do not agree on whether chert and flint are two names for one variety of rock, or whether each is a separate variety. Some, however, now give chert a geological meaning and flint an archaeological meaning. They use the word chert to describe geological formations or rock specimens. They give the name flint to the same rock when it has been used by Indians in making arrowheads, scribers, scrapers, and spearheads.
Quartzite is either a metamorphic rock or a sedimentary rock. (The sedimentary kind of quartzite is described with sand and sandstone on p. 86.) Metamorphic quartzite is made up mostly of quartz. It forms when heat and fluids below the earth’s surface cause the grains and cement of a quartz sandstone to recrystallize. When this happens, the grains interlock and are no longer held together by cement. Metamorphic quartzite, like sedimentary quartzite, is a hard, firm rock that 85 breaks through the quartz grains instead of between them.
Ancient Precambrian metamorphic quartzite occurs at the surface in the Llano uplift area of central Texas, in the Van Horn area of west Texas, and in the Franklin Mountains north of El Paso in extreme west Texas.
Rhyolite is a fine-grained or glassy igneous rock that commonly is extrusive or volcanic. It has a pink, red, tan, white, gray, purple, or black color. This rock, like granite, is made up chiefly of feldspar and a silica mineral, such as quartz, but other minerals may be present. Both rhyolite and granite form from the same kind of molten rock material. Nevertheless, even though their compositions are the same, these two rocks do not look alike. Their textures differ because granite forms slowly and rhyolite forms quickly.
Much of the Texas rhyolite formed from hot, molten lava. This lava flowed out onto the surface either through volcanic cones or cracks in the ground. Some of the lava cooled and hardened too quickly for mineral grains to develop. This rapidly cooled lava formed a rhyolite rock that is made up, at least partly, of glass. In many of the rhyolites, crystalline mineral grains were able to form, but these grains are extremely small, and you may not be able to distinguish them even with a magnifying glass. Some rhyolite, because it hardened from moving, flowing lava, has streaks and bands of different colors and textures. This rhyolite has flow structure.
One variety of rhyolite has easily seen crystals and grains of minerals, such as feldspar, quartz, and mica, scattered through a mass of the tiny crystalline grains (in much the same way that raisins are scattered through a cake). The easily seen crystals and grains are called phenocrysts, and the rock itself is called a rhyolite porphyry.
Many rhyolites and rhyolite porphyries occur in the Tertiary igneous rocks of the Trans-Pecos country of west Texas. Just a few of these localities include the Barrilla Mountains of Jeff Davis and Reeves counties, the Chisos Mountains of Brewster County, the Chinati Mountains of Presidio County, and the Davis Mountains of Jeff Davis County.
Rock Crystal. See Quartz.
Rock Gypsum. See Gypsum.
Rock Salt. See Halite.
Rose Quartz. See Quartz.
Salt. See Halite.
Sand is a loose, uncemented sedimentary deposit made up of fragments of weathered rocks and minerals. These fragments must be of a certain size (between ¹/₁₆ millimeter and 2 millimeters in diameter) in order to be called sand grains. The largest sand grains are about the size of a pinhead. Sand grains are smaller than the fragments known as granules; they are larger than those known as silt.
Many sands are made up chiefly of grains of quartz. This mineral is plentiful and does not easily weather away. In addition, rock fragments and many other minerals, such as feldspar, mica, gypsum, magnetite, and garnet, are found as sand grains.
Rains wash many of the sand grains and other weathered rock and mineral fragments into creeks and rivers. These streams may carry the sand and other sediments long distances before depositing them. Today, we find sands along the banks of many creeks and rivers in Texas and along the beaches of the Gulf of Mexico. The sand in the rivers is in transit to the Gulf. In addition, sand occurs at the surface in other Cenozoic formations and in some of the Paleozoic and Mesozoic formations of Texas.
Sand has many uses. Much building sand, which is used in mortar and concrete, is produced from numerous sand and gravel pits in Texas. Pure quartz sand that 86 can be used to make glass is known as glass sand. Some of it is found in north-central Texas in Lower Cretaceous formations. A large glass sand quarry is located at Santa Anna in Coleman County. Along the Gulf Coastal Plain, sand that is used in glassmaking occurs in Eocene Tertiary strata.
A coarse-grained sand, blast sand, is used with compressed air to clean the walls of brick and stone buildings and to carve designs on monument stones, such as marbles. Some coarse sand is also used as a filtering sand in purifying water. These types of sand have been produced from the Gulf Coastal Plain as well as from other areas of Texas.
Sand grains, when nature cements them together, make up the sedimentary rock sandstone. Some sandstones form when underground water carrying dissolved mineral matter moves through loose sand. As the dissolved mineral matter comes out of solution, it forms a cement that binds the sand grains together.
The cement may be material such as calcite (calcium carbonate), quartz, chalcedony, or opal, which are silica minerals, and limonite and hematite, which are iron oxides. Clay also may serve as a cement. It is either deposited along with the sand or is formed from weathered feldspar sand grains.
The color of the cementing material helps determine the color of the rock. Iron oxide cement, for example, causes the sandstone to have a reddish, yellowish, or brownish color. Sandstones also are white, black, gray, green, or cream colored.
Ordinarily, sandstones break through the cementing material, not through the sand grains. Thus, the broken surface of the rock feels rough and gritty. Some quartz sand grains, however, are tightly cemented with silica to form an extremely hard and compact rock. If this rock breaks smoothly through the grains instead of between them, it is known as quartzite. Some of this sedimentary quartzite occurs in the Texas Gulf Coastal Plain in the Tertiary Catahoula strata. (Another kind of quartzite is described on pp. 84-85.)
Ordinary sandstones are seen at the surface in many localities in Texas, and a number of them have been used as building stones. Some of the places where sandstones occur are in the Cambrian and Pennsylvanian formations of the Llano uplift area of central Texas and in the Pennsylvanian, Permian, and Lower Cretaceous formations of north-central Texas. Tertiary sandstones 87 occur in the Texas Gulf Coastal Plain, and Triassic sandstones are found along the edges of the Texas High Plains. Sandstone is also found in many formations of the Trans-Pecos country of west Texas.
Sandstone. See Sand and Sandstone.
Satin Spar. See Gypsum.
Schist is a metamorphic rock that splits easily along thin, generally parallel layers, called folia. These layers may be either straight or curved, and they are made up of crystalline grains of one or more than one mineral. This structure is called schistosity or foliation. When you examine schist, you will see that many of the mineral grains are flat or long, and that they are lined up in one direction to form the layers. Some schists have fairly large crystals (many with perfect shapes) scattered through them. For example, mica schists may contain beautiful crystals of garnet.
Each kind of schist is named for an outstanding mineral that it contains. Mica schist contains a large amount of mica. We also find hornblende schist, actinolite schist, chlorite schist, talc schist, and graphite schist. (Graphite schist is discussed with graphite on p. 63.)
Schists form from other rocks, such as granite, gabbro, or shale. The rocks are changed into schists by fluids and by heat and pressure below the earth’s surface.
Extremely ancient schists that formed during Precambrian time are exposed at the surface in the Allamoore—Van Horn area of west Texas and in the Llano uplift area of central Texas. Geologists believe that the Packsaddle Schist of the Llano uplift area was once shale. Good exposures of this schist are seen in the Honey Creek area near Packsaddle Mountain in Llano County.
Schorl. See Tourmaline.
Sedimentary Quartzite. See Sand and Sandstone.
Selenite. See Gypsum.
Serpentine is the name given both to a rock and to a mineral. The mineral serpentine (a hydrous magnesium silicate) is found in two different forms. If it is fibrous, it is called chrysotile; if it is layered and platy, it is known as antigorite. Antigorite is brownish green and smooth and waxy looking. Some of it can be split into thin sheets. Chrysotile is made up of greenish, silky fibers, which may be brittle and break apart in large pieces. If, however, the fibers can be pulled apart into soft flexible, little threads, the mineral is called chrysotile asbestos.
Light will pass through both these varieties of serpentine, and both are soft enough to be scratched by a pocket knife. When rubbed across a streak plate, they leave white streaks. Antigorite and chrysotile have no crystal shapes of their own, but several other minerals can alter to form these two varieties of serpentine. Thus antigorite and chrysotile may be found as pseudomorphs in a crystal shape that originally belonged to another mineral.
Antigorite and chrysotile are commonly found closely mixed with dolomite, talc, magnetite, calcite, pyrite, and several other minerals. These minerals make up serpentine rock (also called serpentinite). This rock ordinarily is some shade of green (such as whitish, yellowish, brownish, bluish, or dark blackish green), and it may be mottled. It is brittle or tough and generally is massive. Serpentine rock, like the serpentine minerals, is fairly soft—you can scratch it with a pocket knife.
In the Llano uplift area of central Texas, serpentine rock is found among Precambrian metamorphic rocks, such as gneiss and schist. An especially large deposit in this area is known as the Coal Creek serpentine mass. It is over 3½ miles long, and at one place, it is almost 1½ miles wide. This mass of serpentine extends across the Blanco-Gillespie County line in the extreme northern parts of these two 88 counties. (A little fibrous chrysotile is found here, but it will not break into flexible enough threads to be called chrysotile asbestos.) Several other deposits of serpentine occur in northeastern Gillespie County and in southern Llano County.
It is believed that the Coal Creek serpentine was formed from an igneous rock such as peridotite, which is made up chiefly of grains of the mineral olivine. The peridotite may have been altered into serpentine by underground waters that seeped through it. It is possible, however, that other serpentines in the area were formed when rocks were altered by hot fluids and great pressures far below the earth’s surface.
The Llano area serpentine has been widely used in terrazzo floors. To make these floors, small pieces of serpentine and other colored rocks are put into cement that is spread over a concrete slab. Then, after the cement has hardened, it is ground to a flat, smooth surface and polished. The resulting terrazzo floor is both colorful and durable.
Serpentine rock also is cut into slabs, polished, and used as indoor building stones. Verde antique, a variety often seen in the lobbies of office buildings, consists of green serpentine rock with streaks of white calcite or dolomite in it.
In the Balcones fault zone area (shown on the Texas physiographic outline map, p. 42) from Uvalde County to Williamson County, serpentine occurs with Upper Cretaceous rocks. The serpentine rock is seen at the surface in a few places (such as in Travis and Uvalde counties), but much of it is underground. In several oil fields of this area (as at Thrall field in Williamson County and at Lytton Springs field in Caldwell County), the serpentine rocks contain oil.
Serpentinite. See Serpentine.
Shale is a sedimentary rock made up of tightly packed clay and mud particles. It has a smooth appearance because it is so fine grained. In fact, most of the particles in it are too small to be distinguished with a magnifying glass. These particles are the weathered remains of earlier rocks. They were carried by creeks and rivers to other parts of the land or to the sea, where they formed layers of clay and mud. Later, other sediments were deposited on top of them. The weight of these new sediments squeezed the clays and muds together to form firm, compact shale.
Shale looks very much like some clays. It, like clay, can be almost any color. If the shale contains animal or plant matter, it is black, gray, or blue. If it contains iron oxide (many minerals containing iron alter to this material), it is a shade of red, yellow, or brown. Shale is soft and can be easily scratched by a knife. It also is brittle and crumbles easily. This rock has a property that will help you to distinguish it from clay: the particles that make up the shale were deposited in layers, and the shale splits into flat, thin flakes along these layers, which clay will not do.
Shale is fairly abundant in Texas, especially in Mississippian, Pennsylvanian, and Cretaceous formations. For example, Pennsylvanian shales are found at the surface in north-central Texas, in the area around the Llano uplift of central Texas, and in the Marathon and Solitario uplifts of west Texas.
Many of shale’s uses are the same as those of clay. Some of it can be used to make brick, tile, and other products, and some is often used instead of clay in making portland cement. Cement plants at Dallas, El Paso, Fort Worth, and Waco are located at places where Cretaceous limestones, which also are used in cement making, and Cretaceous shales are found near each other at the surface.
Oil shale, from which petroleum can be obtained by heating, has been found in central Texas. It occurs in Mississippian formations in Lampasas, McCulloch, and San Saba counties. Because oil is much less expensive to obtain from wells, it is not produced from these shales.
Silver has many uses. Like gold, it is a beautiful metal that long has been used for coins and ornaments. A large amount of silver goes to make articles such as spoons, forks, platters, and trays. The photographic industry uses silver—much of the film for cameras is coated with a silver halide. Doctors and dentists use silver, too. The mixture that a dentist uses to fill teeth contains silver along with several other metals. Doctors sometimes use silver wire to fasten broken bones, and silver compounds and solutions, such as silver nitrate, are used in some kinds of medical treatment.
Perhaps more people have heard of legendary, lost silver mines of Texas than of the actual and important silver deposits found in the Trans-Pecos country of west Texas. Some of the west Texas silver minerals include argentite, cerargyrite, and native silver. Although the argentite and native silver commonly found there are mixed with galena, a lead mineral, or with chalcocite, a copper mineral, they also occur separately.
The element silver is found alone as native silver. When pure, it is rather easy to recognize. It is metallic and has a silver-white color that may tarnish to gray, black, or yellowish brown. Native silver is heavy (it has a specific gravity of 10.5) and soft (a pocket knife scratches it easily). When you rub it across a streak plate, native silver, unless it is tarnished, leaves a shiny, silver-white streak. This metal is so ductile that it can be drawn into a wire. It is also malleable and flattens when hit with a hammer.
Silver occurs as crystals, which are poorly shaped cubes and octahedrons, or as irregular masses. It may have a net-like appearance (called reticulate), or it may be shaped like little needles (described then as acicular). It occurs in wires (then called filiform) or as scales or plates.
Prospector.
Two of the Texas silver minerals, argentite and cerargyrite, do not resemble silver at all. 90 Argentite, a silver sulfide, is also called silver glance. It is a dark, lead-gray mineral with a metallic luster that weathers to a dull black. When you rub it across a streak plate, argentite gives a shiny, blackish to lead-gray streak. This mineral is soft enough to leave a mark on paper. It has a specific gravity of 7.3, and it is sectile enough to be cut smoothly (like soap) with a knife. In some places argentite is found as irregular masses or as a coating on rocks and other minerals.
Another silver mineral, cerargyrite (or horn silver) is a silver chloride. This mineral has a nonmetallic luster and is transparent to translucent. It resembles pearl-gray, white, greenish, or colorless wax. When exposed to the light it turns violet brown or black. Cerargyrite is soft—you can scratch it with a fingernail. Like argentite, it is sectile. This mineral has a specific gravity of 5.5, and it commonly occurs as irregular masses and as crusts.
These silver minerals have been mined at a number of places in Trans-Pecos Texas. The largest silver mine in Texas, the Presidio mine, is located near Shafter in south-central Presidio County. It contains argentite, cerargyrite, and native silver, along with galena and several other minerals. This mine is not open now, but in the years between 1885 and 1942, it produced a large amount of silver along with some lead and gold. There are several other lead-silver mines in this Shafter area, but none has produced as much as the Presidio mine.
In this mine, the silver minerals occur mostly in large, flat deposits in Permian limestone and other sedimentary rocks. The minerals are believed to have been deposited there—probably during Tertiary time—by solutions that came from hot magma far below the rocks. As they moved in along the layers of limestone, the solutions replaced portions of this rock with minerals containing silver, lead, and other elements. Later, water seeped into these deposits and dissolved some of the minerals. This dissolved material was then re-deposited, and it formed most of the minerals we now find there.
No silver is being mined in Texas at present, but it has, in the past, been produced from other Trans-Pecos mines. Galena that contains silver (called argentiferous galena) has been mined at the Bird mine at Altuda Mountain (about 14 miles east of Alpine) in northern Brewster County. It also has been obtained from mines in the Quitman Mountains and in the Eagle Mountains of Hudspeth County. Some cerargyrite has been mined at the Plata Verde mine near the Culberson-Hudspeth County line.
Several mines in the Van Horn area of Culberson and Hudspeth counties have produced silver along with copper. An important silver mine in this area is the now idle and flooded Hazel mine. (This mine is described with copper minerals on p. 52.)
Smoky Quartz. See Quartz.
Soapstone. See Talc and Soapstone.
Specular Hematite. See Hematite.
Sulfur is one of Texas’ most valuable minerals. It consists of only a single element, sulfur. This mineral has a resinous luster and is transparent to translucent. Sulfur ordinarily is yellow, but impurities cause it to look greenish, brownish, reddish, or grayish. When you rub it across a streak plate, it leaves a white or a pale-yellow streak. Sulfur has a specific gravity of 2.04 to 2.09 and is soft enough to be scratched by a copper penny. It breaks with a conchoidal to uneven fracture. When it gets hot enough (478° Fahrenheit), sulfur will burn. For this reason, it often is called brimstone.
Sulfur does not conduct electricity and is a poor conductor of heat. You can test how poorly heat passes through it by holding a fragment of sulfur up to your ear. You may be able to hear a crackling sound. The sound results when the outer part of the fragment expands (due to the heat from your hand) while the inner part 91 (which has received no heat) remains unchanged.
Crystals of sulfur are sometimes found, and most of them have either a double-pyramid shape or a flat, tabular shape. Sulfur also occurs as compact masses, as crusts, and as scattered grains.
Native sulfur deposits are found in two widely separated areas of Texas—one in west Texas and the other along the Gulf Coast in southeast Texas, extending over into Louisiana. In the Gulf Coast area, native sulfur is found on some of the salt domes.
The salt domes are huge (from about half a mile to more than 2 miles across), column-shaped masses made up of halite and some anhydrite. These masses have pushed up toward the surface through thousands of feet of sand, clay, and other sedimentary rocks. On top of many of the salt columns is a covering of limestone (calcite), anhydrite, and gypsum known as the cap-rock. It is in this cap-rock that the sulfur is found.
It is thought that when the masses of halite and anhydrite pushed toward the earth’s surface, some of the upper part of the halite dissolved. The anhydrite, however, did not dissolve, and it remained on top of the salt column. Then, a part of this anhydrite was altered into the gypsum, limestone, and sulfur that now are found in some of the cap-rocks. Laboratory experiments have shown that the sulfur in the cap-rocks likely formed through the action of sulfate-reducing bacteria. These bacteria, in the presence of petroleum, converted the sulfate in some of the anhydrite into hydrogen sulfide. Later, hydrogen sulfide was oxidized—perhaps by reaction with more of the anhydrite—to form the sulfur.
Most of the large cap-rock sulfur deposits are about 1,500 to 2,400 feet underground. At first, an attempt was made to get this sulfur out of the ground by digging shafts down to it, but loose, wet, caving sands and poisonous gases, such as hydrogen sulfide, made this mining method almost impossible. Finally, a chemist, Herman Frasch, found a way to obtain the sulfur by making use of sulfur’s low melting point. When sulfur gets slightly hotter than boiling water (235° to 247° Fahrenheit), it melts and becomes a dark, yellowish-brown liquid.
In the Frasch method of sulfur mining, a well is drilled into the salt-dome cap-rock, and three pipes, one inside the other, are put into the well. Superheated water under pressure (hotter than 212° Fahrenheit, the temperature at which water ordinarily turns into steam) is sent down one of the pipes to melt the sulfur in the cap-rock around the bottom of the well. Then, compressed air is sent down another of the pipes. This air presses against the liquid sulfur and forces it up to the surface through the third pipe. At the surface, the sulfur is poured into bins, where it cools and becomes a solid again, or it is transported molten, in pipelines and tankers.
Sulfur has been obtained from a number of the Texas Gulf Coast salt domes including Bryan Mound, Clemens dome, Damon Mound, and Hoskins Mound in Brazoria County; Palangana dome in Duval County; Long Point dome, Nash dome, and Orchard dome in Fort Bend County; High Island dome in Galveston County; Fannett dome and Spindletop dome in Jefferson County; Moss Bluff dome in Liberty County; Gulf dome in Matagorda County; and Boling dome in Wharton County.
In west Texas, sulfur occurs in Permian rocks both at the surface and underground. A small amount of sulfur has been mined in the Rustler Springs area of northeastern Culberson County and northwestern Reeves County, about 50 miles northwest of Pecos. There, scattered grains, crystals, and irregular masses of sulfur occur in cracks and in dissolved-out openings in the Castile Gypsum and in the surface gravel, gypsum, sand, and clay that cover most of this formation.
Sulfur has many uses. It is used as an insect-killer, thus helping our food crops to grow. It is used in pulp and paper manufacturing and in the vulcanizing of rubber. Some other uses are in the making of paints, dyes, and explosives. A large amount of sulfur goes to make sulfuric acid, which itself has numerous uses in the chemical, steel, oil refining, and other industries.
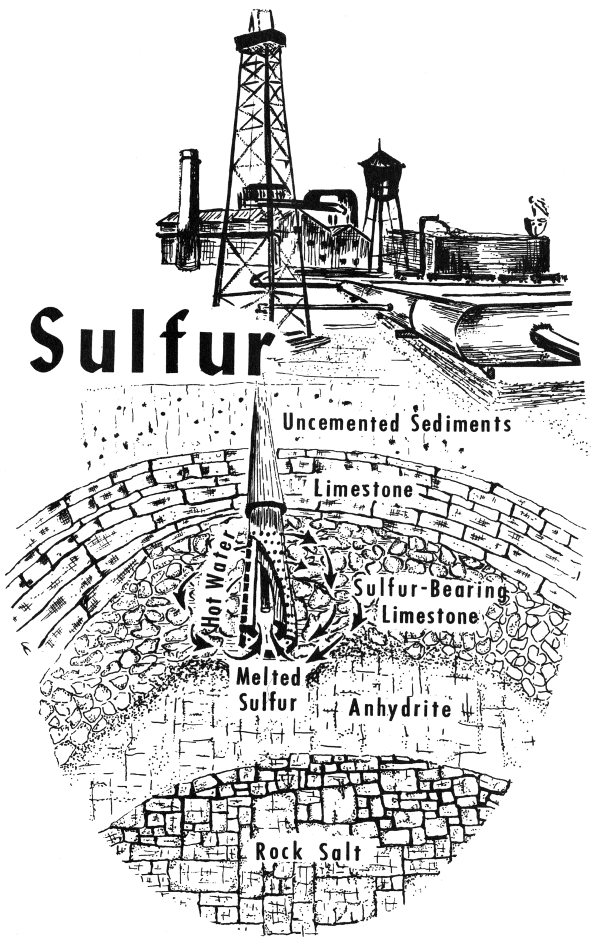
Sulfur is obtained from the cap-rock of Gulf Coastal Plain salt domes by the Frasch process.
Talc, a hydrous magnesium silicate, is an extremely soft mineral—your fingernail scratches it easily. It has a greasy or a pearly luster, and its color is white, light green, or gray. When rubbed across a streak plate, it leaves a white streak.
Talc cleaves perfectly in one direction, and the cleavage fragments are thin, flat, and sheet-like. Its fracture is uneven. This mineral has a soaplike or greasy feel, and it is sectile—a knife will cut through it. Talc is not particularly heavy—it has a specific gravity of 2.7 to 2.8. This mineral seldom occurs with a crystal shape. More commonly it is massive and is granular or layered.
Talc is not always found as a single, pure mineral. In nature, it commonly occurs mixed with one or more other minerals, such as tremolite, anthophyllite, chlorite, and magnetite. This combination of talc with other minerals forms a soft, greasy or soapy-feeling metamorphic rock called soapstone. The talc in this rock may be difficult to identify without special laboratory tests.
In Texas, talc and soapstone are found in Precambrian metamorphic rocks. In west Texas, talc occurs in an area about 20 miles long (just north of U. S. Highway 80 in the vicinity of Allamoore, Eagle Flat siding, and Talc Rock siding) in Hudspeth County. Some of this talc is mined from open pits and used by the ceramic industry to make wall tile. Some of it is finely ground, mixed with insect poison, and used as insect powders and dusts.
Deposits of soapstone, containing talc, occur in the Llano uplift area of central Texas with schist, gneiss, and serpentine 94 rocks in northeastern Gillespie, northwestern Blanco, and southern Llano counties. Smaller deposits occur in northeastern Mason County and in northwestern and southeastern Llano County.
The Llano uplift area soapstones are light green to light buff. It is thought that some of them were once igneous rocks that contained magnesium minerals. Fluids, along with great heat and pressures below the earth’s surface, changed these igneous rocks into soapstone.
Some of this Llano uplift area soapstone is mined from open pits near Willow City in Gillespie County. It is used mostly in making insect powders and roofing granules. In addition, some of the central Texas soapstones have been used for hearths and for fireplace linings.
Topaz, an aluminum fluorosilicate, is a mineral especially prized by collectors because many specimens are gemstones. Topaz is transparent, has a glassy luster, and is quite hard (neither quartz nor a steel file will scratch it). The topaz that has been found in Texas is either colorless, pale blue, or sky blue. This mineral is fairly heavy—its specific gravity is 3.4 to 3.6. It cleaves perfectly in one direction (called basal cleavage), and some of the cleavage fragments have a flat, slabby appearance.
Topaz is commonly found as prism-shaped crystals, as cleavage fragments, and as irregular grains. Some fragments of topaz look like quartz. Topaz, however, is harder and heavier than quartz, and it has perfect basal cleavage, which quartz does not have.
In Texas, crystals, grains, and cleavage fragments of topaz occur in the Llano uplift area of central Texas. They are found near Streeter and Grit in west-central Mason County and near Katemcy in northern Mason County. Here, some of the topaz occurs in Precambrian pegmatite veins that cut through granite rocks. Most of the topaz, however, is found as pebbles in the gravels of nearby creeks, where it has washed after weathering out of the rocks.

Topaz crystal from near Streeter, Mason County, Texas.
Topaz probably originates when hot fluids move up out of molten magma into cracks and cavities in the surrounding rocks. There, the fluids react with elements in the rocks to form the topaz.
Topaz is a good gemstone because, in addition to its beauty, it is hard and is not easily marred by scratches. The Mason County topaz makes excellent gemstones. Most of it is beautiful and clear and is either colorless or of a pleasing blue color. These stones are cut, polished, and mounted in rings and other jewelry. A number of specimens of this Mason County topaz are displayed in museums.
Tourmaline is a complex silicate of boron and aluminum. Other elements, such 95 as magnesium, sodium, lithium, calcium, iron, or fluorine, also may be present. This mineral has a glassy to resinous luster. Only the dark-colored varieties of tourmaline have been found in Texas. One is a black variety called schorl, and another is a brown variety called dravite. Other kinds of tourmaline, although not found in Texas, are colorless or some shade of blue, yellow, red, pink, or green. Some crystals even show more than one color.
Tourmaline is too hard to scratch with a steel file, it has a specific gravity of 3 to 3.25, and it has a conchoidal to uneven fracture. Very little light passes through the dark varieties, and some fragments of schorl look like shiny, black coal.
Tourmaline occurs as masses without crystal shapes, but crystals are commonly found. The crystals are prism-shaped and have small vertical grooves, called striations, on the prism faces. When you look at some crystals from an end, you will see that the cross section is a triangle with the sides bowed outward.
Black tourmaline crystals with milky quartz from north of Llano, Llano County, Texas.
Both the black and the brown varieties of tourmaline have been found at several places in the Llano uplift of central Texas. One well-known locality is at Town Mountain north of Llano in Llano County. Here, the tourmaline occurs in milky quartz that is associated with Precambrian granite rocks. In west Texas, in Culberson and Hudspeth counties, black tourmaline occurs in pegmatite rocks in the Van Horn Mountains, the Carrizo Mountains, and the Wylie Mountains. In the Eagle Mountains of Hudspeth County, it is found in metamorphic rocks as well as in pegmatites.
Some tourmaline formed from hot fluids containing boron that were given off by magmas far below the earth’s surface. These fluids traveled up through cracks and other openings in overlying rocks. As the fluids reacted with other elements and compounds, the tourmaline formed.
The clear, light-colored varieties of tourmaline are much admired, and they are more widely used as gemstones than are the dark-colored varieties. Some collectors, however, find that the dark-colored Texas tourmalines, when cut and polished, make shiny, attractive gemstones.
Some tourmaline is used as grinding material, but no Texas tourmaline is produced for this purpose.
Travertine. See Calcite.
In 1945, the world suddenly became aware of the awesome power of atomic energy when the element uranium was used to produce some of the first atomic bombs. Uranium does not occur alone in nature but is found combined with other elements in a number of minerals.
All of the uranium minerals are radioactive. The uranium they contain is gradually breaking down and changing into a series of 13 other elements, called daughter elements. Each daughter element breaks down and changes into the next daughter element of the series. While breaking down, these elements give off particles and rays of energy.
This energy or radioactivity is made up of what are called alpha particles, beta particles, and gamma rays. You cannot see, hear, taste, smell, or feel them. The 96 alpha and beta particles are weak and do not travel far. The gamma rays, however, can travel farther and can pass through seemingly solid material. Scientists have found that these rays can move through about 1 foot of rock, 2½ feet of water, and several hundred feet of air.
Prospectors searching for uranium minerals carry instruments that are able to detect this radioactivity. The uranium itself gives off only alpha particles, but some of its daughter elements give off gamma rays. These daughter elements are normally found with the uranium, and it is their strong gamma rays that the instruments are most apt to detect.
A Geiger counter is used to detect radioactivity.
One of the instruments used is the Geiger counter. It indicates radioactivity by means of a meter, a flashing light, or a clicking sound, which can be heard through earphones. Another instrument for detecting radioactivity is the scintillation counter. It is more sensitive than the Geiger counter and it can detect radioactivity from a greater distance. The scintillation counter can be used from an automobile or an airplane, but the Geiger counter must be quite 97 close to the source of radioactivity to be of use.
Various uranium minerals have been found, mostly in small amounts, in a number of places in Texas. Some of these minerals, such as uraninite or pitchblende, are heavy and dark colored. Others, including carnotite, tyuyamunite, autunite, and uranophane, are a shade of yellow or green. They are quite soft. Deposits of the light-colored uranium minerals have been mined from two areas of Texas. One of these areas is in Garza County on the Texas High Plains, and the other is in Karnes and Live Oak counties in the Gulf Coastal Plain.
One of the light-colored uranium minerals, carnotite, is a potassium-uranium vanadate, which has a bright canary-yellow or lemon-yellow color. This mineral is transparent to translucent and has an earthy or a pearly luster. Carnotite usually is found as crusts and as powdery masses. It is quite soft and can be scratched with a fingernail.
Carnotite, along with tyuyamunite, autunite, and several other soft, yellowish or greenish uranium minerals, is found in the Texas Gulf Coastal Plain. These minerals occur in the Jackson, Catahoula, and Oakville strata (which are Tertiary in age) in an area extending from Gonzales County to the Rio Grande (in parts of the area indicated by no. 2 and no. 3 on the geologic map, pp. 4-5). The largest deposits in this district have been found in the Karnes County area.
The Gulf Coastal Plain uranium minerals occur mostly with sandstones and clays in a sequence of strata that contains volcanic ash. It is believed that small scattered amounts of uranium compounds that were present in the volcanic ash sediments were dissolved by seeping underground water. These waters then moved into the sandstones and clays where they deposited the uranium as carnotite and as other uranium minerals.
Another uranium mineral, uranophane (calcium-uranium silicate), also occurs in Texas. Uranophane has a yellow to yellow-orange color and a pearly to greasy luster. When rubbed across a streak plate, it leaves a light yellow to a light yellow-orange streak. It is soft enough to be scratched by a copper penny. Uranophane has been found in extrusive igneous rocks in northwestern Presidio County in west Texas.
A dark-colored uranium mineral, pitchblende, is a variety of the mineral uraninite, uranium dioxide. Pitchblende does not occur with a crystal shape but rather as rounded and irregular-shaped masses. It is brownish black, greenish black, or black. If you rub it across a streak plate, pitchblende leaves a brownish-black streak. This mineral is heavy (it has a specific gravity of 6.5 to 8.5) and hard (a pocket knife will not scratch it, although a steel file will). Pitchblende has a submetallic luster and looks dull, greasy, or like pitch or tar.
Small amounts of pitchblende have been found at several places in Texas. One of these localities is a few miles west of Burnet in Burnet County in central Texas. Here, the pitchblende occurs in Precambrian igneous rocks that are associated with gneiss. In south Texas, some fine, scattered particles of pitchblende have been found about 325 feet below the surface in Tertiary (Pliocene) sediments that cover the Palangana salt dome in Duval County. No pitchblende is mined in Texas.
Uranophane. See Uranium Minerals.
Vitrophyre. See Obsidian and Vitrophyre.
Volcanic ash deposits, which also are known as pumicite, are loose and powdery. They are made up mostly of material that is thrown into the air when volcanoes erupt. If a volcano erupts with a violent explosion, the nearby rocks are blown into powder. Molten lava also is hurled into the air, where some of it immediately cools to become tiny bubbles and particles of glass. The winds may carry some of this fine material far away before depositing it.
Deposits of volcanic ash are white, 98 bluish, greenish, yellowish, or grayish, and some of them glisten like snow in the sunlight. They feel rough and gritty. When examined under a microscope, this material shows the tiny curved and sharp-cornered particles of the broken volcanic glass. Deposits of volcanic ash may also contain clay, silt, sand, or other impurities.
Volcanoes, which may have been located in the Davis Mountains and in other areas of west Texas and in northern Mexico, erupted during Tertiary time. The volcanic ash that we find at the surface today in some of the Tertiary formations in Texas could have come from these volcanoes. Tertiary volcanic ash deposits occur in the Texas Gulf Coastal Plain (such as in Brazos, Fayette, Karnes, Polk, Starr, Trinity, and other counties) and in the Trans-Pecos country of west Texas.
Volcanic ash deposits of Quaternary (Pleistocene) age, which are less than a million years old, are found in a number of counties on the Texas High Plains. Farther to the east, ash deposits occur in Baylor, Dickens, Kent, and Wilbarger counties. This volcanic ash may have come from a volcano that erupted in northern New Mexico during Quaternary time.
Volcanic ash or pumicite has several commercial uses. Some is used to make pozzolan cement, and some is used in sweeping compounds, cleansing and scouring powders, and abrasive soaps. Pumicite has been mined in Dickens, Scurry, Starr, and several other counties of Texas.
Wad. See Manganese Minerals.
Wood Opal. See Opal.
For convenient reference, the Texas minerals described in this book are listed below, together with their chemical compositions, specific gravities, and hardness. You will be able to find similar information about additional minerals in mineralogy textbooks such as those noted on page 24.
| Mineral | Composition | Specific Gravity | Hardness |
| Albite | NaAlSi₃O₈ | 2.62 | 6 |
| Almandite | Fe₃Al₂ (SiO₄)₂ | 4.2 | 7 |
| Amphibole asbestos | Ca₂Mg₅Si₈O₂₂(OH)₂ | 3.0-3.3 | 1-2½ |
| Anhydrite | CaSO₄ | 2.9 | 3-3½ |
| Argentite | Ag₂S | 7.3 | 2-2½ |
| Azurite | Cu₃(CO₃)₂(OH)₂ | 3.77 | 3½-4 |
| Barite | BaSO₄ | 4.5 | 3-3½ |
| Biotite | K(Mg, Fe)₃AlSi₃O₁₀(OH)₂ | 2.8-3.2 | 2½-3 |
| Braunite | 3MnMnO₃MnSiO₃ | 4.75-4.82 | 6-6½ |
| Calcite | CaCO₃ | 2.72 | 3 |
| Carnotite | K₂O·2UO₃·V₂O₅·nH₂O | 5.03 | 2 |
| Cassiterite | SnO₂ | 6.8-7.1 | 6-7 |
| Celestite | SrSO₄ | 3.95-3.97 | 3-3½ |
| Cerargyrite | AgCl | 5.5 | 1-1½ |
| Chalcocite | Cu₂S | 5.5-5.8 | 2½-3 |
| Chalcopyrite | CuFeS₂ | 4.1-4.3 | 3½-4 |
| Cinnabar | HgS | 8.10 | 2½ |
| Dolomite | CaMg(CO₃)₂ | 2.85 | 3½-4 |
| Feldspar (see Albite, Microcline, Orthoclase) | |||
| Fluorite | CaF₂ | 3.18 | 4 |
| Galena | PbS | 7.4-7.6 | 2½ |
| Garnet (see Almandite, Grossularite) | |||
| Gold | Au | 15.0-19.3 | 2½-3 |
| Graphite | C | 2.2 | 1-2 |
| Grossularite | Ca₃Al₂(SiO₄)₃ | 3.53 | 6½ |
| Gypsum | CaSO₄·2H₂O | 2.32 | 2 |
| Halite | NaCl | 2.16 | 2½ |
| Hematite | Fe₂O₃ | 5.26 | 1-6½ |
| Hollandite | MnBaMn₁₆O₁₄ | 4.7-5 | 4-6 |
| Limonite | FeO(OH)·nH₂O | 3.6-4.0 | 1-5½ |
| Magnetite | Fe₃O₄ | 5.18 | 6 |
| Malachite | Cu₂CO₃(OH)₂ | 3.9-4.03 | 3½-4 |
| Mica (see Muscovite, Biotite) | |||
| Microcline | KAlSi₃O₈ | 2.54-2.57 | 6 |
| Muscovite | KAl₃Si₃O₁₀(OH)₂ | 2.76-3.1 | 2-2½ |
| Opal | SiO₂·nH₂O | 1.9-2.2 | 5-6 |
| Orthoclase | KAlSi₃O₈ | 2.57 | 6 |
| Pitchblende | UO₂ | 6.5-8.5 | 5½ |
| Pyrite | FeS₂ | 5.02 | 6-6½ |
| Pyrolusite | MnO₂ | 4.75 | 1-2 |
| Quartz | SiO₂ | 2.65 | 7 |
| Serpentine | Mg₃Si₂O₅(OH)₄ | 2.48 | 3-4 |
| Silver | Ag | 10.5 | 2½-3 |
| Sulfur | S | 2.05-2.09 | 1½-2½ |
| Talc | Mg₃Si₄O₁₀(OH)₂ | 2.7-2.8 | 1 |
| Topaz | Al₂SiO₄(F,OH)₂ | 3.4-3.6 | 8 |
| Tourmaline | Complex silicate of boron and aluminum | 3.0-3.25 | 7-7½ |
| Uranophane | CaO·2UO₃·2SiO₂·7H₂O | 3.8-3.9 | 2-3 |
Many books have been written about rocks and minerals. Some are listed below, and it is likely that your librarian will be able to suggest others.
Getting Acquainted With Minerals, by George L. English and David E. Jensen. McGraw-Hill Book Company, Inc., New York, N. Y. (second edition, 1958).
The Rock Book, by Carroll L. Fenton and Mildred A. Fenton. Doubleday & Company, Inc., Garden City, N. Y. (1940).
Mineral Collector’s Guide, by David E. Jensen. Ward’s Natural Science Establishment, Inc., Rochester, N. Y. (1953).
My Hobby is Collecting Rocks and Minerals, by David E. Jensen. Hart Book Company, New York, N. Y. (1955).
Rocks and Minerals, by Richard M. Pearl. Barnes & Noble, New York, N. Y. (1956).
1001 Questions Answered About the Mineral Kingdom, by Richard M. Pearl. Dodd, Mead & Company, New York, N. Y. (1959).
Rocks and Minerals, by Herbert S. Zim and Paul R. Schaffer. Simon and Schuster, Inc., New York, N.Y. (1957).
Economic Mineral Deposits, by Alan M. Bateman. John Wiley & Sons, Inc., New York, N. Y. (second edition, 1950).
A Textbook of Mineralogy, by Edward S. Dana, revised by William E. Ford. John Wiley & Sons, Inc., New York, N. Y. (fourth edition, 1932).
Industrial Minerals and Rocks (Nonmetallics Other Than Fuels), Joseph L. Gillson, Editor-in-Chief. The American Institute of Mining, Metallurgical, and Petroleum Engineers, New York, N. Y. (third edition, 1960).
Dana’s Manual of Mineralogy, revised by Cornelius S. Hurlbut, Jr. John Wiley & Sons, Inc., New York, N. Y. (seventeenth edition, 1959).
Mineralogy, by Edward H. Kraus, Walter F. Hunt, and Lewis S. Ramsdell. McGraw-Hill Book Company, Inc., New York, N. Y. (fifth edition, 1959).
Nonmetallic Minerals, by Raymond B. Ladoo and W. M. Meyers. McGraw-Hill Book Company, Inc., New York, N. Y. (second edition, 1951).
Rocks and Rock Minerals, by Louis V. Pirsson, revised by Adolph Knopf. John Wiley and Sons, Inc., New York, N. Y. (third edition, 1947).
A Field Guide to Rocks and Minerals, by Frederick H. Pough. Houghton Mifflin Company, Boston, Mass. (third edition, 1960).
Mineral Facts and Problems, by the Staff of the Bureau of Mines. U. S. Bureau of Mines Bulletin 585. U. S. Government Printing Office, Washington, D. C. (1960).
Entries marked with an asterisk are published by the Bureau of Economic Geology, The University of Texas, Austin. Those not out of print are distributed at nominal sale price, and a list of publications will be sent on request. These publications can be consulted at many public libraries and Chamber of Commerce offices.
*Report on the Pavitte Silver-Copper Prospect in Burnet County, Texas, by V. E. Barnes. Univ. Texas, Bureau Econ. Geol. Mineral Resource Survey Circ. 5 (1936).
*Report on the Sheridan Copper Prospect in Burnet County, Texas, by V. E. Barnes. Univ. Texas, Bur. Econ. Geol. Mineral Resource Survey Circ. 9 (1936).
*Building Stones of Central Texas, by V. E. Barnes, R. F. Dawson, and G. A. Parkinson. Univ. Texas Pub. 4246 (1947).
*Iron Ore in the Llano Region, Central Texas, by V. E. Barnes. Univ. Texas, Bur. Econ. Geol. Rept. Inves. No. 5 (1949).
*Utilization of Texas Serpentine, by V. E. Barnes, D. A. Shock, and W. A. Cunningham. Univ. Texas Pub. 5020 (1950).
*Lead Deposits in the Upper Cambrian of Central Texas, by V. E. Barnes. Univ. Texas, Bureau Econ. Geol. Rept. Inves. No. 26 (1956).
*Mineral Resources of the Colorado River Industrial Development Association Area, by J. W. Dietrich and J. T. Lonsdale. Univ. Texas, Bureau Econ. Geol. Rept. Inves. No. 37 (1958).
*Some Uranium Occurrences in West Texas, by D. H. Eargle. Univ. Texas, Bureau Econ. Geol. Rept. Inves. No. 27 (1956).
*A Preliminary Report on the Stratigraphy of the Uranium-Bearing Rocks of the Karnes County Area, South-Central Texas, by D. H. Eargle and J. L. Snider. Univ. Texas, Bureau Econ. Geol. Rept. Inves. No. 30 (1957).
The Brown Iron Ores of Eastern Texas, by E. B. Eckel. U. S. Geol. Survey Bull. 902 (1938).
*The Rustler Springs Sulphur Deposits as a Source of Fertilizer, by G. L. Evans. Univ. Texas, Bureau Econ. Geol. Rept. Inves. No. 1 (1946).
Origin of the Gulf Coast Salt-Dome Sulphur Deposits, by Herbert W. Feely and J. Lawrence Kulp. Bull. Amer. Assoc. Petrol. Geol., vol. 41, pp. 1802-1853 (1957).
*Pegmatites of the Van Horn Mountains, Texas, by P. T. Flawn. Univ. Texas, Bureau Econ. Geol. Rept. Inves. No. 9 (1951).
*The Hazel Copper-Silver Mine, Culberson County, Texas, by P. T. Flawn. Univ. Texas, Bureau Econ. Geol. Rept. Inves. No. 16 (1952).
*Basement Rocks of Texas and Southeast New Mexico, by P. T. Flawn. Univ. Texas Pub. 5605 (1956).
*Texas Miners Boost Talc Output, by P. T. Flawn. Univ. Texas, Bureau Econ. Geol. Rept. Inves. No. 35 (1958).
*Geology and Mineral Deposits of Pre-Cambrian Rocks of the Van Horn Area, Texas, by P. B. King and P. T. Flawn. Univ. Texas Pub. 5301 (1953).
*Igneous Rocks of the Balcones Fault Region of Texas, by J. T. Lonsdale. Univ. Texas Bull. 2744 (1927).
Mineral Resources of the Llano-Burnet Region, Texas, with an Account of the Pre-Cambrian Geology, by Sidney Paige. U. S. Geol. Survey Bull. 450 (1911).
*Mineral Resources of the Texas Coastal Plain (Preliminary Report), by J. M. Perkins and J. T. Lonsdale. Univ. Texas, Bureau Econ. Geol. Mineral Resource Circ. 38 (1955).
Geology and Ore Deposits of the Shafter Mining District, Presidio County, Texas, by C. P. Ross. U. S. Geol. Survey Bull. 928-B (1943).
*The Geology of Texas, Vol. II, Structural and Economic Geology, by E. H. Sellards, C. L. Baker, and others. Univ. Texas Bull. 3401 (1935).
*Texas Mineral Resources, by E. H. Sellards and others. Univ. Texas Pub. 4301 (1946).
*Geological Resources of the Trinity River Tributary Area in Texas and Oklahoma, by H. B. Stenzel, A. E. Weissenborn, and others. Univ. Texas Pub. 4824 (1948).
Uranium at Palangana Salt Dome, Duval County, Texas, by A. D. Weeks and D. H. Eargle. In U. S. Geol. Survey Prof. Paper 400-B (1960).
Geology of the Quicksilver Deposits of the Terlingua District, Texas, by R. G. Yates and G. A. Thompson. U. S. Geol. Survey Prof. Paper 312 (1959).
Amorphous—without crystalline structure and therefore without regular form.
Balcones fault zone—a system of faults extending from north of Waco in McLennan County, through Travis and Bexar counties, to near Del Rio in Val Verde County (see p. 42).
Boulder—a large rock or mineral fragment that has a diameter greater than 256 millimeters (about 10 inches).
Breccia—a rock made up of sharp-cornered, cemented fragments with diameters greater than 2 millimeters (about ⁸/₁₀₀ of an inch).
Cambrian—the earliest period of the Paleozoic Era (see p. 3).
Cenozoic—the present era, one of the great divisions of geologic time (see p. 3). This era began about 63 million years ago.
Clastic—made up of broken fragments of rocks or minerals.
Cleavage—occurs when minerals split along smooth flat surfaces that are parallel to possible crystal faces. These planes as well as crystal faces are controlled by the crystal lattice or atomic structures of the minerals.
Cleavage fragment—a mineral specimen that has been broken along its planes of cleavage.
Cobble—a rock or mineral fragment that has a diameter between 64 and 256 millimeters (about 2½ and 10 inches).
Conchoidal—a curved fracture surface shaped like the inside of a shell or spoon.
Conglomerate—a rock composed of cemented, rounded rock or mineral fragments, most of which are of gravel size.
Cretaceous—the third and latest period of the Mesozoic Era (see p. 3).
Cryptocrystalline—made up of tiny crystalline particles that are too small to be distinguished even under high magnification.
Crystalline—having a definite, orderly internal structure.
Cube—a solid that has six equal, square sides.
Dodecahedron—a solid that has twelve plane, four-sided faces.
Element—a basic building block of all matter, which cannot be separated into different substances by ordinary chemical means.
Eocene—the second epoch of the Tertiary Period (see p. 3).
Epoch—a unit of geologic time that is a subdivision of a period.
Era—a major division of geologic time, which consists of several periods.
Extrusive rocks—igneous rocks formed from magma that was extruded on the earth’s surface.
Fault—a break in the rocks or strata of the earth’s crust along which movement or slippage has taken place.
Fluid—a substance made up of particles that can move freely about; it can be a liquid or a gas.
Formation—rocks or strata that are recognized and mapped as a unit.
Fracture—the kind of surface obtained if a mineral is broken in a different direction from that of the cleavage or parting. Commonly, fracture surfaces are rough, uneven, or curved, whereas cleavage surfaces are smooth.
Geologic map (areal)—shows the extent and distribution of formations exposed at the earth’s surface.
Granular—the texture of a rock or mineral that is made up of visible grains. If all the grains are about the same size, the term equigranular is used.
Granule—a rock or mineral fragment that has a diameter of from 2 to 4 millimeters (about ⁸/₁₀₀ to ¹⁵/₁₀₀ of an inch).
Gravel—uncemented rock or mineral fragments that have diameters greater than 2 millimeters (about ⁸/₁₀₀ of an inch).
Gulf Coastal Plain—an area that extends, in Texas, from the Gulf of Mexico to the Balcones fault zone and in which Quaternary, Tertiary, and Upper Cretaceous strata crop out at the surface (see p. 42).
High Plains—an area in northwest Texas extending from the Pecos River valley north to the Oklahoma-Texas boundary (see p. 42).
Igneous rocks—rocks formed by the cooling and hardening of hot, molten rock material.
Intrusive rocks—igneous rocks that have formed below the surface of the earth.
Lava—molten rock material that has poured out onto the earth’s surface from volcanoes; also the rock that is formed after the molten material has cooled and hardened.
Llano uplift—an area in central Texas where Precambrian and early Paleozoic rocks occur at the earth’s surface (see p. 42).
Magma—hot, molten rock material from which igneous rocks form.
Massive—in a mass, without a regular or complete form.
Mesozoic—an era, one of the great divisions of geologic time (see p. 3). This era began about 230 million years ago and lasted until about 63 million years ago.
Metamorphic rock—rock formed from igneous or sedimentary rocks that are altered by heat, pressure, and fluids below the earth’s surface.
Miocene—the fourth epoch of the Tertiary Period (see p. 3).
Mississippian—the fifth period of the Paleozoic Era (see p. 3).
Nodule—a small, rounded mass or lump.
Octahedron—a solid that has eight triangular faces.
Opaque—no light can pass through.
Ordovician—the second period of the Paleozoic Era (see p. 3).
Paleozoic—an era, one of the great divisions of geologic time (see p. 3). This era began at the end of Precambrian time and lasted until about 230 million years ago.
Parting—occurs when a mineral breaks along a flat surface that is not a true cleavage plane.
Pebble—a rock or mineral fragment that has a diameter between 4 and 64 millimeters (about ¹⁵/₁₀₀ and 2½ inches).
Pennsylvanian—the sixth period of the Paleozoic Era (see p. 3).
Period—a unit of geologic time, a subdivision of an era.
Permian—the last period of the Paleozoic Era (see p. 3).
Physiographic outline map—shows location of natural regions (p. 42).
Playa lake—a temporary shallow lake in a nearly level, closed basin, which has no drainage outlet.
Pleistocene—the first epoch of the Quaternary Period (see p. 3).
Pliocene—the last epoch of the Tertiary Period (see p. 3).
Precambrian—comprises the Early and the Late Precambrian Eras, the earliest great divisions of geologic time. Rocks that formed more than 600 million years ago are known as Precambrian rocks.
Pyritohedron—a solid that has twelve 5-sided faces.
Quaternary—the present period of geologic time; the second period of the Cenozoic Era (see p. 3).
Recent—the present epoch of geologic time; the second epoch of the Quaternary Period (see p. 3).
Sectile—describes material, such as soap, that can be cut smoothly with a knife.
Sediments—material deposited by water, wind, or ice on the earth’s surface.
Sedimentary rocks—rocks made up of sediments.
Series—a subdivision of a system that includes all rocks formed during an epoch.
Specific gravity—the ratio of the weight of a substance to the weight of an equal volume of water.
Streak—the color of the powder of a mineral.
System—all rocks formed during a period.
Tertiary—the first period of the Cenozoic Era (see p. 3).
Translucent—light will pass through, but objects cannot be seen.
Transparent—light will pass through, and objects can be seen.
Trans-Pecos—area of Texas located west of the Pecos River (see p. 42).
Volcanic rocks—igneous rocks that have formed on the earth’s surface; extrusive rocks.
A B C D E F G H I J K L M N O P Q R S T U V W X Y Z
End of Project Gutenberg's Texas Rocks and Minerals, by Roselle M. Girard
*** END OF THIS PROJECT GUTENBERG EBOOK TEXAS ROCKS AND MINERALS ***
***** This file should be named 52839-h.htm or 52839-h.zip *****
This and all associated files of various formats will be found in:
http://www.gutenberg.org/5/2/8/3/52839/
Produced by Stephen Hutcheson, Dave Morgan and the Online
Distributed Proofreading Team at http://www.pgdp.net
Updated editions will replace the previous one--the old editions will
be renamed.
Creating the works from print editions not protected by U.S. copyright
law means that no one owns a United States copyright in these works,
so the Foundation (and you!) can copy and distribute it in the United
States without permission and without paying copyright
royalties. Special rules, set forth in the General Terms of Use part
of this license, apply to copying and distributing Project
Gutenberg-tm electronic works to protect the PROJECT GUTENBERG-tm
concept and trademark. Project Gutenberg is a registered trademark,
and may not be used if you charge for the eBooks, unless you receive
specific permission. If you do not charge anything for copies of this
eBook, complying with the rules is very easy. You may use this eBook
for nearly any purpose such as creation of derivative works, reports,
performances and research. They may be modified and printed and given
away--you may do practically ANYTHING in the United States with eBooks
not protected by U.S. copyright law. Redistribution is subject to the
trademark license, especially commercial redistribution.
START: FULL LICENSE
THE FULL PROJECT GUTENBERG LICENSE
PLEASE READ THIS BEFORE YOU DISTRIBUTE OR USE THIS WORK
To protect the Project Gutenberg-tm mission of promoting the free
distribution of electronic works, by using or distributing this work
(or any other work associated in any way with the phrase "Project
Gutenberg"), you agree to comply with all the terms of the Full
Project Gutenberg-tm License available with this file or online at
www.gutenberg.org/license.
Section 1. General Terms of Use and Redistributing Project
Gutenberg-tm electronic works
1.A. By reading or using any part of this Project Gutenberg-tm
electronic work, you indicate that you have read, understand, agree to
and accept all the terms of this license and intellectual property
(trademark/copyright) agreement. If you do not agree to abide by all
the terms of this agreement, you must cease using and return or
destroy all copies of Project Gutenberg-tm electronic works in your
possession. If you paid a fee for obtaining a copy of or access to a
Project Gutenberg-tm electronic work and you do not agree to be bound
by the terms of this agreement, you may obtain a refund from the
person or entity to whom you paid the fee as set forth in paragraph
1.E.8.
1.B. "Project Gutenberg" is a registered trademark. It may only be
used on or associated in any way with an electronic work by people who
agree to be bound by the terms of this agreement. There are a few
things that you can do with most Project Gutenberg-tm electronic works
even without complying with the full terms of this agreement. See
paragraph 1.C below. There are a lot of things you can do with Project
Gutenberg-tm electronic works if you follow the terms of this
agreement and help preserve free future access to Project Gutenberg-tm
electronic works. See paragraph 1.E below.
1.C. The Project Gutenberg Literary Archive Foundation ("the
Foundation" or PGLAF), owns a compilation copyright in the collection
of Project Gutenberg-tm electronic works. Nearly all the individual
works in the collection are in the public domain in the United
States. If an individual work is unprotected by copyright law in the
United States and you are located in the United States, we do not
claim a right to prevent you from copying, distributing, performing,
displaying or creating derivative works based on the work as long as
all references to Project Gutenberg are removed. Of course, we hope
that you will support the Project Gutenberg-tm mission of promoting
free access to electronic works by freely sharing Project Gutenberg-tm
works in compliance with the terms of this agreement for keeping the
Project Gutenberg-tm name associated with the work. You can easily
comply with the terms of this agreement by keeping this work in the
same format with its attached full Project Gutenberg-tm License when
you share it without charge with others.
1.D. The copyright laws of the place where you are located also govern
what you can do with this work. Copyright laws in most countries are
in a constant state of change. If you are outside the United States,
check the laws of your country in addition to the terms of this
agreement before downloading, copying, displaying, performing,
distributing or creating derivative works based on this work or any
other Project Gutenberg-tm work. The Foundation makes no
representations concerning the copyright status of any work in any
country outside the United States.
1.E. Unless you have removed all references to Project Gutenberg:
1.E.1. The following sentence, with active links to, or other
immediate access to, the full Project Gutenberg-tm License must appear
prominently whenever any copy of a Project Gutenberg-tm work (any work
on which the phrase "Project Gutenberg" appears, or with which the
phrase "Project Gutenberg" is associated) is accessed, displayed,
performed, viewed, copied or distributed:
This eBook is for the use of anyone anywhere in the United States and
most other parts of the world at no cost and with almost no
restrictions whatsoever. You may copy it, give it away or re-use it
under the terms of the Project Gutenberg License included with this
eBook or online at www.gutenberg.org. If you are not located in the
United States, you'll have to check the laws of the country where you
are located before using this ebook.
1.E.2. If an individual Project Gutenberg-tm electronic work is
derived from texts not protected by U.S. copyright law (does not
contain a notice indicating that it is posted with permission of the
copyright holder), the work can be copied and distributed to anyone in
the United States without paying any fees or charges. If you are
redistributing or providing access to a work with the phrase "Project
Gutenberg" associated with or appearing on the work, you must comply
either with the requirements of paragraphs 1.E.1 through 1.E.7 or
obtain permission for the use of the work and the Project Gutenberg-tm
trademark as set forth in paragraphs 1.E.8 or 1.E.9.
1.E.3. If an individual Project Gutenberg-tm electronic work is posted
with the permission of the copyright holder, your use and distribution
must comply with both paragraphs 1.E.1 through 1.E.7 and any
additional terms imposed by the copyright holder. Additional terms
will be linked to the Project Gutenberg-tm License for all works
posted with the permission of the copyright holder found at the
beginning of this work.
1.E.4. Do not unlink or detach or remove the full Project Gutenberg-tm
License terms from this work, or any files containing a part of this
work or any other work associated with Project Gutenberg-tm.
1.E.5. Do not copy, display, perform, distribute or redistribute this
electronic work, or any part of this electronic work, without
prominently displaying the sentence set forth in paragraph 1.E.1 with
active links or immediate access to the full terms of the Project
Gutenberg-tm License.
1.E.6. You may convert to and distribute this work in any binary,
compressed, marked up, nonproprietary or proprietary form, including
any word processing or hypertext form. However, if you provide access
to or distribute copies of a Project Gutenberg-tm work in a format
other than "Plain Vanilla ASCII" or other format used in the official
version posted on the official Project Gutenberg-tm web site
(www.gutenberg.org), you must, at no additional cost, fee or expense
to the user, provide a copy, a means of exporting a copy, or a means
of obtaining a copy upon request, of the work in its original "Plain
Vanilla ASCII" or other form. Any alternate format must include the
full Project Gutenberg-tm License as specified in paragraph 1.E.1.
1.E.7. Do not charge a fee for access to, viewing, displaying,
performing, copying or distributing any Project Gutenberg-tm works
unless you comply with paragraph 1.E.8 or 1.E.9.
1.E.8. You may charge a reasonable fee for copies of or providing
access to or distributing Project Gutenberg-tm electronic works
provided that
* You pay a royalty fee of 20% of the gross profits you derive from
the use of Project Gutenberg-tm works calculated using the method
you already use to calculate your applicable taxes. The fee is owed
to the owner of the Project Gutenberg-tm trademark, but he has
agreed to donate royalties under this paragraph to the Project
Gutenberg Literary Archive Foundation. Royalty payments must be paid
within 60 days following each date on which you prepare (or are
legally required to prepare) your periodic tax returns. Royalty
payments should be clearly marked as such and sent to the Project
Gutenberg Literary Archive Foundation at the address specified in
Section 4, "Information about donations to the Project Gutenberg
Literary Archive Foundation."
* You provide a full refund of any money paid by a user who notifies
you in writing (or by e-mail) within 30 days of receipt that s/he
does not agree to the terms of the full Project Gutenberg-tm
License. You must require such a user to return or destroy all
copies of the works possessed in a physical medium and discontinue
all use of and all access to other copies of Project Gutenberg-tm
works.
* You provide, in accordance with paragraph 1.F.3, a full refund of
any money paid for a work or a replacement copy, if a defect in the
electronic work is discovered and reported to you within 90 days of
receipt of the work.
* You comply with all other terms of this agreement for free
distribution of Project Gutenberg-tm works.
1.E.9. If you wish to charge a fee or distribute a Project
Gutenberg-tm electronic work or group of works on different terms than
are set forth in this agreement, you must obtain permission in writing
from both the Project Gutenberg Literary Archive Foundation and The
Project Gutenberg Trademark LLC, the owner of the Project Gutenberg-tm
trademark. Contact the Foundation as set forth in Section 3 below.
1.F.
1.F.1. Project Gutenberg volunteers and employees expend considerable
effort to identify, do copyright research on, transcribe and proofread
works not protected by U.S. copyright law in creating the Project
Gutenberg-tm collection. Despite these efforts, Project Gutenberg-tm
electronic works, and the medium on which they may be stored, may
contain "Defects," such as, but not limited to, incomplete, inaccurate
or corrupt data, transcription errors, a copyright or other
intellectual property infringement, a defective or damaged disk or
other medium, a computer virus, or computer codes that damage or
cannot be read by your equipment.
1.F.2. LIMITED WARRANTY, DISCLAIMER OF DAMAGES - Except for the "Right
of Replacement or Refund" described in paragraph 1.F.3, the Project
Gutenberg Literary Archive Foundation, the owner of the Project
Gutenberg-tm trademark, and any other party distributing a Project
Gutenberg-tm electronic work under this agreement, disclaim all
liability to you for damages, costs and expenses, including legal
fees. YOU AGREE THAT YOU HAVE NO REMEDIES FOR NEGLIGENCE, STRICT
LIABILITY, BREACH OF WARRANTY OR BREACH OF CONTRACT EXCEPT THOSE
PROVIDED IN PARAGRAPH 1.F.3. YOU AGREE THAT THE FOUNDATION, THE
TRADEMARK OWNER, AND ANY DISTRIBUTOR UNDER THIS AGREEMENT WILL NOT BE
LIABLE TO YOU FOR ACTUAL, DIRECT, INDIRECT, CONSEQUENTIAL, PUNITIVE OR
INCIDENTAL DAMAGES EVEN IF YOU GIVE NOTICE OF THE POSSIBILITY OF SUCH
DAMAGE.
1.F.3. LIMITED RIGHT OF REPLACEMENT OR REFUND - If you discover a
defect in this electronic work within 90 days of receiving it, you can
receive a refund of the money (if any) you paid for it by sending a
written explanation to the person you received the work from. If you
received the work on a physical medium, you must return the medium
with your written explanation. The person or entity that provided you
with the defective work may elect to provide a replacement copy in
lieu of a refund. If you received the work electronically, the person
or entity providing it to you may choose to give you a second
opportunity to receive the work electronically in lieu of a refund. If
the second copy is also defective, you may demand a refund in writing
without further opportunities to fix the problem.
1.F.4. Except for the limited right of replacement or refund set forth
in paragraph 1.F.3, this work is provided to you 'AS-IS', WITH NO
OTHER WARRANTIES OF ANY KIND, EXPRESS OR IMPLIED, INCLUDING BUT NOT
LIMITED TO WARRANTIES OF MERCHANTABILITY OR FITNESS FOR ANY PURPOSE.
1.F.5. Some states do not allow disclaimers of certain implied
warranties or the exclusion or limitation of certain types of
damages. If any disclaimer or limitation set forth in this agreement
violates the law of the state applicable to this agreement, the
agreement shall be interpreted to make the maximum disclaimer or
limitation permitted by the applicable state law. The invalidity or
unenforceability of any provision of this agreement shall not void the
remaining provisions.
1.F.6. INDEMNITY - You agree to indemnify and hold the Foundation, the
trademark owner, any agent or employee of the Foundation, anyone
providing copies of Project Gutenberg-tm electronic works in
accordance with this agreement, and any volunteers associated with the
production, promotion and distribution of Project Gutenberg-tm
electronic works, harmless from all liability, costs and expenses,
including legal fees, that arise directly or indirectly from any of
the following which you do or cause to occur: (a) distribution of this
or any Project Gutenberg-tm work, (b) alteration, modification, or
additions or deletions to any Project Gutenberg-tm work, and (c) any
Defect you cause.
Section 2. Information about the Mission of Project Gutenberg-tm
Project Gutenberg-tm is synonymous with the free distribution of
electronic works in formats readable by the widest variety of
computers including obsolete, old, middle-aged and new computers. It
exists because of the efforts of hundreds of volunteers and donations
from people in all walks of life.
Volunteers and financial support to provide volunteers with the
assistance they need are critical to reaching Project Gutenberg-tm's
goals and ensuring that the Project Gutenberg-tm collection will
remain freely available for generations to come. In 2001, the Project
Gutenberg Literary Archive Foundation was created to provide a secure
and permanent future for Project Gutenberg-tm and future
generations. To learn more about the Project Gutenberg Literary
Archive Foundation and how your efforts and donations can help, see
Sections 3 and 4 and the Foundation information page at
www.gutenberg.org
Section 3. Information about the Project Gutenberg Literary Archive Foundation
The Project Gutenberg Literary Archive Foundation is a non profit
501(c)(3) educational corporation organized under the laws of the
state of Mississippi and granted tax exempt status by the Internal
Revenue Service. The Foundation's EIN or federal tax identification
number is 64-6221541. Contributions to the Project Gutenberg Literary
Archive Foundation are tax deductible to the full extent permitted by
U.S. federal laws and your state's laws.
The Foundation's principal office is in Fairbanks, Alaska, with the
mailing address: PO Box 750175, Fairbanks, AK 99775, but its
volunteers and employees are scattered throughout numerous
locations. Its business office is located at 809 North 1500 West, Salt
Lake City, UT 84116, (801) 596-1887. Email contact links and up to
date contact information can be found at the Foundation's web site and
official page at www.gutenberg.org/contact
For additional contact information:
Dr. Gregory B. Newby
Chief Executive and Director
gbnewby@pglaf.org
Section 4. Information about Donations to the Project Gutenberg
Literary Archive Foundation
Project Gutenberg-tm depends upon and cannot survive without wide
spread public support and donations to carry out its mission of
increasing the number of public domain and licensed works that can be
freely distributed in machine readable form accessible by the widest
array of equipment including outdated equipment. Many small donations
($1 to $5,000) are particularly important to maintaining tax exempt
status with the IRS.
The Foundation is committed to complying with the laws regulating
charities and charitable donations in all 50 states of the United
States. Compliance requirements are not uniform and it takes a
considerable effort, much paperwork and many fees to meet and keep up
with these requirements. We do not solicit donations in locations
where we have not received written confirmation of compliance. To SEND
DONATIONS or determine the status of compliance for any particular
state visit www.gutenberg.org/donate
While we cannot and do not solicit contributions from states where we
have not met the solicitation requirements, we know of no prohibition
against accepting unsolicited donations from donors in such states who
approach us with offers to donate.
International donations are gratefully accepted, but we cannot make
any statements concerning tax treatment of donations received from
outside the United States. U.S. laws alone swamp our small staff.
Please check the Project Gutenberg Web pages for current donation
methods and addresses. Donations are accepted in a number of other
ways including checks, online payments and credit card donations. To
donate, please visit: www.gutenberg.org/donate
Section 5. General Information About Project Gutenberg-tm electronic works.
Professor Michael S. Hart was the originator of the Project
Gutenberg-tm concept of a library of electronic works that could be
freely shared with anyone. For forty years, he produced and
distributed Project Gutenberg-tm eBooks with only a loose network of
volunteer support.
Project Gutenberg-tm eBooks are often created from several printed
editions, all of which are confirmed as not protected by copyright in
the U.S. unless a copyright notice is included. Thus, we do not
necessarily keep eBooks in compliance with any particular paper
edition.
Most people start at our Web site which has the main PG search
facility: www.gutenberg.org
This Web site includes information about Project Gutenberg-tm,
including how to make donations to the Project Gutenberg Literary
Archive Foundation, how to help produce our new eBooks, and how to
subscribe to our email newsletter to hear about new eBooks.